Antioxidant Activity of Surinamese Medicinal Plants with Adaptogenic Properties and Correlation with Total Phenolic Contents
Abstract
Plant-based preparations are commonly used in Suriname (South America) as adaptogens. In this study, fifteen alleged adaptogenic Surinamese plants have been assessed for their antioxidant activity (AA), total phenolic contents (TPC), and total flavonoid contents (TFC). The investigated plants were Anacardium occidentale, Spondiasdulcis, Annona muricata, Euterpe oleracea, Oenocarpus bacaba, Luffa acutangula, Punicagranatum, Malpighia emarginata, Syzygiumaqueum, Syzygiumcumini, Averrhoa carambola, and Renealmiaalpinia (fruit); Hibiscus sabdariffa (calyx); as well as Aloe vera and Cestrum latifolium (leaf). Aqueous extracts (1 - 3,000 μg/ mL) were prepared. AA was determined by the FRAP and the DPPH assay. TPC and TFC were determined by the Folin-Ciocalteu’s and an AlCl3 colorimetric method, respectively, using gallic acid (GA) and rutin (R), respectively, as standards. Data are means ± SDs (n ≥ 3; P < 0.05). FRAP values and DPPH-scavenging activities correlated positively with each other and with TPC but not with TFC. The preparations from M. emarginata, A. carambola, A. occidentale, O. bacaba, C. latifolium, and H. sabdariffa displayed the highest FRAP values (54 ± 14 to 412 ± 30 µM Fe2+/100 μg), DPPH-scavenging activities (IC50 values of 33 ± 14 to 250 ± 50 μg/mL), and TPC (51 ± 4 to 280 ± 78 µM GAE/100 µg). TFC of all samples were ≤ 10 ± 3 RE/100 µg. The adaptogenic properties of these plants may (partially) be attributed to their high content of antioxidant phenolic compounds and may make them candidates of novel sources of health-promoting antioxidants.
Author Contributions
Academic Editor: Jie Yin, Institute of Subtropical Agriculture and University of Chinese Academy of Sciences, china
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Dennis R.A. Mans, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The dependence of humans on oxygen for their metabolism leads to the continuous formation of reactive oxygen-derived species (ROS) in the body as by-products of reactions involving oxygen 1. ROS can be generated from either endogenous or exogenous sources. Endogenous sources of ROS are cellular organelles where oxygen consumption is high, such as mitochondria, peroxisomes, and endoplasmic reticulum 2. Exogenous sources of ROS are hazardous environmental chemicals which, as shown for the alkylating antineoplastic agent cyclophosphamide, produce free radicals during their metabolic conversion (see, for instance 3). Furthermore, in individuals with inherited erythrocyte glucose-6-phosphate dehydrogenase deficiency 4, the red blood cells provide insufficient NADPH in response to the rate of ROS formation, resulting in the accumulation of ROS, damage to the red blood cells, and hemolytic anemia (see, for instance, 5).
Examples of ROS are superoxide radical anion, hydrogen peroxide, peroxyl radicals, and hydroxyl 1. These species play important roles in key physiological functions such as cell signaling and homeostasis 6, 7. However, ROS can also attack cellular macromolecules like the nuclear DNA and plasma membrane lipids causing cellular injury 8. Fortunately, the body has both enzymatic antioxidant systems (for instance, superoxide dismutase, catalase, and glutathione peroxidase) and non-enzymatic mechanisms (for instance, bilirubin and albumin) to help mitigate this damage 9. However, when ROS overwhelm these physiological defenses, oxidative stress occurs 10. Oxidative stress can lead to lipid peroxidation, cell and tissues toxicity, and several types of genetic damage that eventually could cause genotoxicity, mutagenicity, secondary cancers, and even cell death (see, for instance 11). The resulting homeostatic disruption of multiple metabolic processes may eventually result in the development of neoplastic, cardiovascular, diabetic, neurodegenerative, age-related, and inflammatory ailments 10, 12.
In addition to innate defense systems, exogenous antioxidants provided through the diet and/or nutritional supplements may help protect the body from oxidative stress 13. Indeed, several studies have suggested that the consumption of compounds rich in antioxidants decreases the risk of developing the above-mentioned diseases 14, 15. An important class of plant-derived antioxidants is represented by phenolic compounds, secondary plant metabolites made up of one or more aromatic ring(s) coupled to one or more hydroxyl group(s) 16. Phenolic compounds also help protect plants from pathogens, animal and insect attack, as well as ultraviolet radiation; provide plants their characteristic colors; and contribute to the organoleptic properties of plants 17. There are tens of thousands of plant phenolic compounds including the main dietary constituents flavonoids, phenolic acids, and tannins, in addition to coumarins, naphthoquinones, stilbenes, anthraquinones, and lignans 13, 16. Their mitigating effect on oxidative stress has been attributed to their ability to eliminate potentially harmful oxidizing free radical species by acting as reducing agents, hydrogen donors, quenchers of singlet oxygen, or chelators of metal ions that catalyze oxidation reactions 13, 16.
Not surprisingly, the interest in plant-derived phenolic compounds with antioxidant activity is on the rise, and many of these compounds are promoted for preventing and treating illnesses and maintaining general well-being (see, for instance, 18). Compounds used for the latter purpose have been called adaptogens, an unofficial term that refers to herbal substances that would help fight stress and fatigue and stimulate well-being by increasing the body’s ability to adapt and survive 19. When considering the importance of antioxidants to human health 13, 14, 15 and the capacity of plant phenolic compounds to act as antioxidants 13, 16, these phytochemicals may well constitute important ingredients of adaptogens.
The traditional use of plants and plant-based preparations is deeply rooted in the Republic of Suriname (South America), despite the nationwide availability of affordable and accessible allopathic forms of medicine 20. Many traditional preparations are also used for promoting general health, to fight stress, and to obtain extra health benefits 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, and can therefore be regarded as adaptogens. Thus, these substances may display unusually high antioxidant activity and contain unusually high amounts of phenolic compounds. So far, studies dealing with these subjects have not been carried out. Therefore, it was decided to assess a number of Surinamese plant preparations that may qualify as adaptogens for their antioxidant activity in vitro and their total phenolic content. As flavonoids represent an important class of plant phenolics that are also able to scavenge free radicals and inactivate catalytic metal ions 31, the plant samples have also been evaluated for their total flavonoid content. The results obtained may provide scientific substantiation for the adaptogenic properties of the plants and may help identify novel natural sources of antioxidants.
Materials and Methods
Plant selection and preparation of Plant Extracts
The plants evaluated in the current study are mentioned in Table 1. They have been selected on the basis of the number of times they have been dealt with in well-known publications on the use of medicinal plants in Suriname 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. The plants have been collected in the period between September and November 2019 in rural areas around Suriname’s capital city Paramaribo that had been free from herbicidal or pesticidal use for at least the preceding six months. The collected plants have been authenticated by staff members from the National Herbarium of Suriname. The plant parts of interest (Table 1) were washed with distilled water, air-dried, washed again, macerated, and extracted with distilled water. This was based on the traditional custom to prepare herbal medicinal teas, infusions, and decoctions by extracting, brewing, or boiling leaves, fruits, stembark, roots, or other plant parts with water 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. The extracts were filtered, concentrated by freeze-drying, and the material obtained was divided in aliquots of 0.2 g which were stored at -20 oC and tested shortly thereafter.
Table 1. Plants investigated in the current study, plant part used and method of processing, as well as the most common traditional medical uses in Suriname| Plant family | Plant part used and method of processing | Most common traditional adaptogenic uses (references) | ||
| Anacardiaceae | Anacardium occidentaleL. (cashew; kasyu) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Throat infections 23, 24, 26, 28 | |
| Anacardiaceae | Spondiasdulcis L.(ambarella; pommesitère) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Fever, cough, wounds, sores, burns 22, 25 | |
| Annonaceae | Annona muricata L.(soursop; zuurzak) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Insomnia, tension, anxiety, exam stress, bedwetting 24, 26, 28, 29 | |
| Arecaceae | Euterpe oleracea Mart.(açai; podosiri) | Fruit; pulp around seeds removed, macerated, and extracted with distilled water at room temperature, filtered, and freeze-dried | Anemia, low blood pressure 29, 30 | |
| Arecaceae | Oenocarpus bacaba Mart.(turu palm; kumbu) | Fruit; pulp around seeds removed, macerated, and extracted with distilled water at room temperature, filtered, and freeze-dried | Anemia, low blood pressure 28, 29 | |
| Asphodelaceae | Aloe vera (L.) Burm.f.(aloe; aloë) | Inner leaves; squeezed, and gel diluted with distilled water at room temperature, filtered, and freeze-dried | Burns, scars, wound infections, skin rash, scars, hair loss, dandruff, fever, headache 24, 26, 28, 29, 30 | |
| Cucurbitaceae | Luffa acutangula (L.) Roxb.(ridged gourd; sukwa) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Gall bladder functioning 24 | |
| Lythraceae | Punicagranatum L.(pomegranate; granaatappel) | Fruit; seed pulps removed, macerated, and extracted with distilled water at room temperature, filtered, and freeze-dried | General health tonic, bleeding gums, lower respiratory tract complaints, diarrhoea 21, 24, 28 | |
| Malpighiaceae | Malpighia emarginata DC. (1753)(acerola; Westindische kers) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Flu, sore throat, pimples 28, 29 | |
| Malvaceae | Hibiscus sabdariffa L.(roselle; syuru) | Calyces; macerated, and infusion prepared, filtered, and freeze-dried | Coughing, microbial infections, skin and hair care 30 | |
| Myrtaceae | Syzygiumaqueum (Burm.f.) Alston(watery rose apple; pommerak) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Tonic to improve liver and brain functioning 22, 25 | |
| Myrtaceae | Syzygiumcumini (L.) Skeels. (jambolan; dyamun) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Anemia, abdominal pain, diarrhoea, coughing up of blood 23, 24, 26, 28 | |
| Oxalidaceae | Averrhoa carambola L.(star fruit; birambi) | Fruit; squeezed, and juice collected at room temperature, filtered, and freeze-dried | Fever, respiratory tract complaints, fungal skin infections 28 | |
| Solanaceae | Cestrum latifolium Lam.(bitter greens; bitawiwiri) | Leaves; macerated and extracted with water for 1 h at 70 oC, filtered, and freeze-dried | Anemia, migraine, stress, flu, eye inflammation, sore throat, pimples, itching 24, 28, 29 | |
| Zingiberaceae | Renealmiaalpinia (Rottb.) Maas (ink plant; masusa) | Fruit; pulp extracted at room temperature, filtered, and freeze-dried | Genital steam baths 27 | |
Drugs and Chemicals
Iron(III) chloride hexahydrate (FeCl3 . 6H2O), iron(II) sulfate heptahydrate (FeSO4 . 7H2O), and 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), Folin-Ciocalteu reagent, gallic acid, aluminum chloride hexahydrate (AlCl3 . 6H2O), rutin, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were from Sigma-Aldrich (St. Louis, MO, USA). Ethanol was from Applichem GmbH (Darmstadt, Germany), sodium carbonate (Na2CO3) from Merck, (Darmstadt, Germany), and sodium acetate (CH3COONa) from BDH Laboratory Supplies (Poole, UK). All other chemicals used were from our laboratory stock and were of the highest grade available.
Determination of Antioxidant Activity of Plant Extracts by the Ferric Reducing/Antioxidant Power Assay
The antioxidant activity of the plant extracts was determined by a spectrophotometric method based on the ability of an antioxidant to reduce a ferric (Fe3+) ion from the Fe3+-TPTZ complex to the ferrous (Fe2+) ion from a Fe2+-tripyridyltriazine complex through the donation of an electron at low pH 32. The reactions were spectrophotometrically monitored by measuring the change from the colorless Fe3+-TPTZ complex to the intensely blue-colored Fe2+-tripyridyltriazine complex at a wavelength at 593 nm. Thus, 3 mL freshly prepared ferric reducing/antioxidant power (FRAP) reagent was mixed with 100 µL of a plant extract and 1 mL distilled water. The FRAP reagent consisted of TPTZ 10 mM in HCl, FeCl3 . 6H2O 20 mM, and acetate buffer 300 mM pH 3.6 in the proportion of 1/1/10 (v/v/v).
After thorough mixing and incubation for 30 min in the dark and at room temperature, the absorbance at 593 nm was recorded against a blank consisting of samples where the plant extract was substituted by distilled water. The change in absorbance was directly related to the total reducing power of the electron-donating antioxidants present in the plant extracts. These were estimated from a calibration curve constructed from the absorbance of different concentrations of FeSO4 at 593 nm and expressed as µm Fe2+ equivalents reduced per 100 μg lyophilized plant extract.
Determination of Antioxidant Activity of Plant Extracts by the 1,1-diphenyl-2-picrylhydrazyl Assay
The plant extracts were also assessed for antioxidant activity using a DPPH free radical scavenging assay 33. This assay is based on the ability of an antioxidant to inactivate the stable DPPH cation free radical following donation of an electron or hydrogen atom. During his process, the violet colored DPPH molecule becomes colorless to pale yellow, which can spectroscopically be monitored at a wavelength 517 nm. Thus, for each plant extract, seven serial dilutions between 1 and 3,000 μg/mL were prepared, and 0.3 mL of each dilution was mixed with 3 mL absolute ethanol and 0.5 mL DPPH solution of 0.5 mM in ethanol. After 90 min in the dark and at room temperature, the absorbance of the solutions was measured at 517 nm against a mixture of 3.3 mL ethanol and 0.5 mL sample as a blank. The control solution consisted of 3.5 mL ethanol and 0.3 mL DPPH solution.
The percentage antioxidant activity (AA %) of each dilution of each plant extract was determined using the formula:
where Abssample is the absorbance of the plant extract, Absblank the absorbance of the blank, and Abscontrol the absorbance of the control. For each plant extract, the absorbance values of the dilutions were plotted against the corresponding concentrations. From the resulting dose-response curve, IC50 values were derived, i.e., the concentrations of the plant extracts (in μg/mL) accomplishing a 50% decrease in absorbance when compared to untreated controls. The lower the IC50 value, the higher the antioxidant activity.
Determination of Total Phenolic Content of Plant Extracts
The total phenolic content of the extracts was determined using the Folin-Ciocalteu’s method 34. The Folin-Ciocalteu reagent is a mixture of phosphomolybdate and phosphotungstate, and the method is based on the transfer of electrons in alkaline medium from phenolic compounds to the phosphomolybdate/phosphotungstate complex to form a blue chromophore that is spectrophotometrically detectable. Thus, each plant extract was dissolved in distilled water to a concentration of 100 μg/mL. Of each extract, an aliquot of 1.0 mL was added to 0.1 mL Folin-Ciocalteu reagent 1 N, after which 0.9 mL distilled water was added. The mixture was shaken and allowed to react for 5 min at room temperature. Then, 1.0 mL of Na2CO3 7% (w/v) was added. This solution was adjusted with distilled water to a final volume of 3.4 mL and thoroughly mixed. After incubation for 30 min in the dark, the absorbance was read at 765 nm with respect to a blank containing only Folin-Ciocalteu reagent 1 N and Na2CO3 7% (w/v). The total phenolic content of the plant extracts was calculated from the linear equation of a standard curve prepared with gallic acid (1 to 200 μg/mL) and expressed as µM gallic acid equivalents (GAE) per 100 g lyophilized plant extract.
Determination of Total Flavonoid Content of Plant Extracts
Total flavonoid content of the plant extracts was determined using a previously described aluminum chloride (AlCl3) colorimetric method 35. This method is based on the formation of acid-stable complexes between AlCl3 and the hydroxyl groups of flavones and flavonols. Thus, each plant extract was dissolved in distilled water to give samples of 100 μg/mL. A volume of 0.5 mL AlCl3 2% (w/v) in absolute ethanol was added to 0.5 mL aliquots of each sample, after which 0.5 mL 1 M potassium acetate and 0.5 mL 1 M HCL were added. The mixture was incubated for 10 min at room temperature and the absorbance was measured at 425 nm against a blank of distilled water. A yellow color indicated the presence of flavonoids. Total flavonoid content of the plant extracts was calculated by intrapolation into a standard curve of rutin prepared from serial dilutions of this compound between 0 and 200 µg/L. Data were expressed as mg rutin equivalents (RE) per 100 g lyophilized plant extract.
Data Processing and Statistics
All experiments have been carried out at least three times in triplicate. Based on the degree of antioxidant activity found, the samples have been classified into those with high, intermediate, and low antioxidant activity. Data (means ± SDs) have been compared using Student’s t test. The relationship between FRAP values and DPPH free radical-scavenging activities, and between FRAP values or DPPH free radical-scavenging activities and total phenolic contents or total flavonoid contents, were explored using two-tailed analysis of bivariate correlation. In all cases, P values < 0.05 were taken to indicate statistically significant differences.
Results
Relationships Between FRAP Values and DPPH free Radical-scavenging Activities, and Between Antioxidant Activities and Phytochemical Contents of the Plant Samples
In the current study, fifteen plant extracts that are used in Suriname as adaptogens have been assessed for their antioxidant activity, total phenolic content, and total flavonoid content. Using linear regression analysis, a significant positive correlation (p value < 0.001) was found between FRAP values and DPPH free radical-scavenging activities (a correlation coefficient R2 of about 0.30; Figure 1).
Figure 1.Relationship between DPPH-scavenging activities and FRAP values in the plant extracts
Particularly FRAP values of the preparations correlated well with their total phenolic contents (correlation coefficient R2 of about 0.91; Figure 2a), those with higher activity having a relatively high phenolic content and those with low activity a relatively low phenolic content (p value < 0.001). Such a good correlation was not found between DPPH free radical-scavenging activities and total phenolic contents, but there was still a significant positive relationship (p value < 0.001) between both parameters (a correlation coefficient R2 of about 0.25; Figure 2b).
Figure 2a.Relationship between total phenolic contents and FRAP values in the plant extracts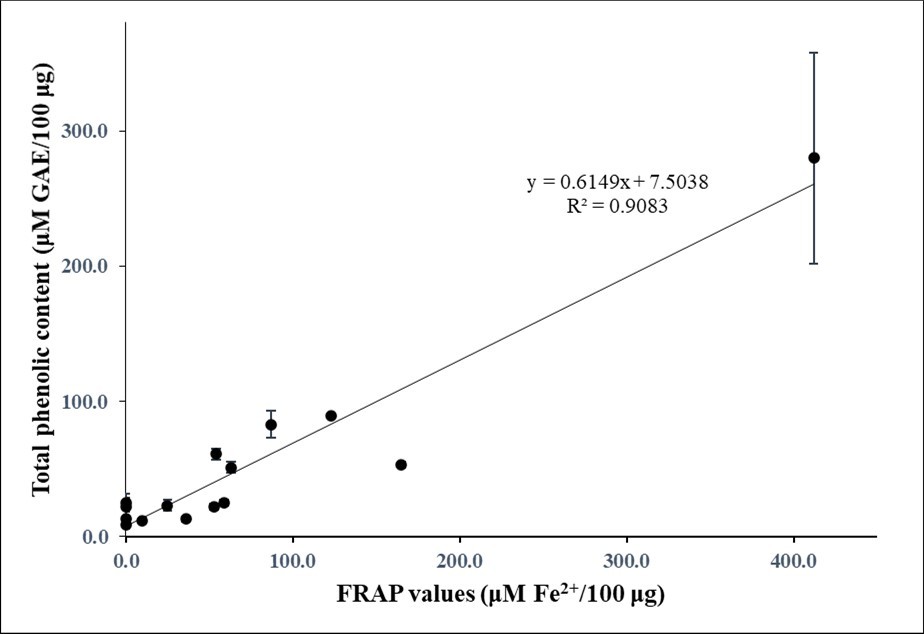
Figure 2b.Relationship between total phenolic contents and DPPH-scavenging activities in the plant extracts
On the other hand, FRAP values and DPPH free radical-scavenging activities did not correlate well with total flavonoid contents. Correlation coefficients R2 were 0.0012 and 0.0092, respectively (Figure 3a and Figure 3b, respectively), indicating a poor correlation between antioxidant activities and total flavonoid contents (p values of 0.904 and 0.594, respectively).
Figure 3a.Relationship between total flavonoid contents and FRAP values in the plant extracts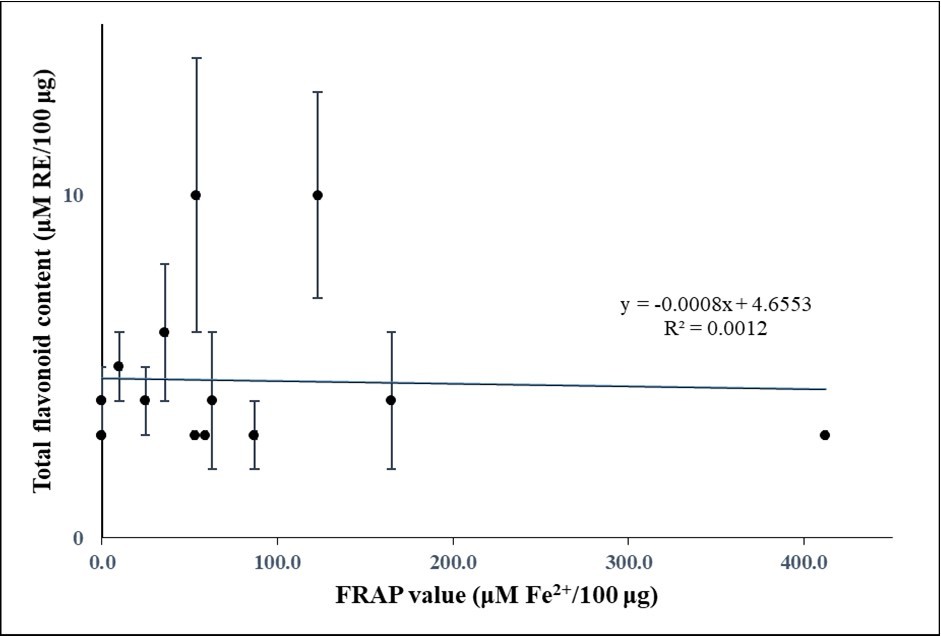
Figure 3b.Relationship between total flavonoid contents and DPPH-scavenging activities in the plant extracts
FRAP Values and DPPH Free Radical-scavenging Activities, and Total Phenolic and Flavonoid Contents of the Plant Samples
Based on the degree of antioxidant activity found, the samples have been classified into those with high, intermediate, and low antioxidant activity (Table 2).
Table 2. FRAP values, DPPH-scavenging activities, total phenolic contents, and total flavonoid contents of the plant extracts investigated in the current study| Plant species | FRAP activity(µm Fe2+ equivalents reduced per 100 μg lyophilized plant extract) | DPPH activity(IC50 in μg/mL) | Total phenolic content (µM GAE per 100 µg lyophilized plant extract) | Total flavonoid content (µM RE per100 µg lyophilized plant extract) |
| High antioxidant activity | ||||
| M. emarginata | 412 ± 30 | 33 ± 14 | 280 ± 78 | 3 ± 0 |
| Intermediate antioxidant activity | ||||
| O. bacaba | 165 ± 29 | 78 ± 17 | 53 ± 2 | 4 ± 2 |
| A. carambola | 123 ± 13 | 133 ± 14 | 89 ± 1 | 10 ± 3 |
| C. latifolium | 87 ± 17 | 150 ± 0 | 83 ± 10 | 3 ± 1 |
| H. sabdariffa | 63 ± 9 | 183 ± 29 | 51 ± 4 | 4 ± 2 |
| A. occidentale | 54 ± 14 | 250 ± 50 | 61 ± 4 | 10± 4 |
| Low antioxidant activity | ||||
| E. oleracea | 59 ± 12 | 617 ± 29 | 25 ± 2 | 3 ± 0 |
| P. granatum | 53 ± 4 | 400 ± 0 | 22 ± 2 | 3 ± 0 |
| S. aqueum | 36 ± 8 | 2,533 ± 351 | 13 ± 2 | 6 ± 2 |
| R. alpinia | 25 ± 5 | > 3,000 | 23 ± 4 | 4 ± 1 |
| S. dulcis | 10 ± 3 | > 3,000 | 12 ± 2 | 5 ± 1 |
| S. cumini | 0 | 308 ± 8 | 25 ± 4 | 3 ± 0 |
| A. vera | 0 | 1,850 ± 650 | 22 ± 10 | 3 ± 0 |
| L. acutangula | 0 | > 3,000 | 9 ± 3 | 4 ± 1 |
| A. muricata | 0 | > 3,000 | 13 ± 5 | 4 ± 1 |
Plant Extracts with high Antioxidant Activity
The Malpighia emarginata DC fruit extract exhibited the highest antioxidant activity of the 15 plant samples evaluated, i.e., a FRAP value of µM Fe2+ equivalents reduced per 100 μg lyophilized material and a DPPH free radical-scavenging activity at an IC50 value of 33 ± 14 μg/mL (Table 2). This preparation also had the highest total phenol content, namely 280 ± 78 µM GAE per 100 µg lyophilized plant material (Table 2). However, its total flavonoid content was relatively low (3 ± 0 RE per 100 µg lyophilized material; Table 2). Thus, the relatively high antioxidant activity of the M. emarginata preparation correlated well with its relatively high total phenolic content but not with its relatively low total flavonoid content.
Plant Extracts with an Intermediate Antioxidant Activity
The extracts from Averrhoa carambola L., Anacardium occidentale L., and Oenocarpus bacaba Mart. fruit as well as those from Hibiscus sabdariffa L calyx and Cestrum latifolium Lam. leaf had intermediate to high FRAP values (54 ± 14 to 165 ± 29 µM Fe2+ equivalent reduced per 100 μg lyophilized material, respectively; Table 2), and relatively high DPPH free radical-scavenging activities (IC50 values of 78 ± 17 to 250 ± 50 μg/mL; Table 2). When compared to the M. emarginata sample, these preparations had the second highest total phenolic content, namely 51 ± 4 to 83 ± 10 µM GAE per 100 µg lyophilized plant material (Table 2). Their total flavonoid contents ranged from were 3 ± 1 to 10 ± 3 µM RE per 100 µg lyophilized plant material (Table 2). Thus, the fairly high antioxidant activity of these preparations correlated reasonably well with their intermediate to high total phenolic content but not with their total flavonoid content.
Plant Extracts with a Low Antioxidant Activity
The extracts from Euterpe oleracea Mart., Aloe vera (L.) Burm.f., Punicagranatum L., Syzygiumcumini L., Renealmiaalpinia (Rottb.), Spondiasdulcis L., Annona muricata L., Luffa acutangula (L.) Roxb, and Syzygiumaqueum (Burm.f.) exhibited very low to intermediate FRAP values (0 to 59 ± 12 µM Fe2+ equivalents reduced per 100 μg lyophilized material; Table 2) and very low to high DPPH free radical-scavenging activities (IC50 values of > 3000 to 308 ± 8 μg/mL; Table 2). Their total phenolic content was on the lower side (9 ± 3 to 25 ± 4 GAE per 100 µg lyophilized plant material; Table 2). Their total flavonoid content ranged from 3 ± 0 to 6 ± 2 µM RE per 100 µg lyophilized material; Table 2). Apparently, the antioxidant activity of these samples partially correlated with their total phenolic content but not very well with their total flavonoid content.
Discussion
Preparations from A. occidentale, S. dulcis, A. muricata, E. oleracea, O. bacaba, L. acutangula, P. granatum, M. emarginata, S. aqueum, S. cumini, A. carambola, and R. alpinia fruit; H. sabdariffa calyx; as well as A. vera and C. latifolium leaf are extensively used in Suriname for their presumed adaptogenic properties 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. In this study, the possibility that a relatively high antioxidant activity and phenolic content are involved in the alleged health-promoting properties of the plants has been investigated using FRAP and DPPH assays as well as Folin-Ciocalteu’s and AlCl3 colorimetric methods. The results obtained showed a good correlation between FRAP values and DPPH free radical-scavenging activities of the samples, as well as linear relationships between antioxidant activities and total phenolic content. Such a relationship was not found with flavonoid content. Furthermore, the samples from M. emarginata, A. carambola, A. occidentale, and O. bacaba fruit as well as C. latifolium leaf and H. sabdariffa calyx displayed both intermediate to high antioxidant activities and intermediate to high phenolic contents. These findings may account, at least partially, for the presumed adaptogenic properties of these plants. This did not seem to hold true for the samples of S. dulcis, A. muricata, E. oleracea, L. acutangula, P. granatum, S. aqueum, S. cumini, and R. alpinia fruit as well as that from A. vera leaf. Thus, these plants either cannot be considered ‘genuine’ adoptogens, or their adoptogenic qualities may be attributable to properties other than the capacity to eliminate free radicals.
The reasonable correlation between FRAP values and DPPH free radical-scavenging activity suggested that both activities were to some degree consistent with each other. This is in accordance with the comparable principles of these assays: the FRAP assay is based on the ability of an antioxidant to reduce Fe3+ ions to Fe2+ ions by donating a hydrogen atom 32, the DPPH assay on the capacity of an antioxidant to inactivate the stable DPPH cation radical by donating a hydrogen atom or electron 33. Therefore, it can be suggested that the antioxidant ingredients in the different plant samples may have some structural and/or biochemical characteristics in common. Indeed, the possibility of structure-activity-relationships accounting for these observations has been mentioned before (see, for instance 36).
The statistically significant positive relationship of both FRAP values and DPPH free radical-scavenging activities with total phenolic contents suggests that phenolics played an important role in the antioxidant activity of the plant samples. This is in accordance with data from many previous studies (see, for instance, references 37, 38) suggesting that the antioxidant activities of plant samples were to a considerable extent determined by their phenolic content. On the other hand, the absence of a significant positive relationship of either FRAP values and DPPH free radical-scavenging activities with total flavonoid contents suggests that these ingredients were not major contributors to the antioxidant activities of the plant extracts. Of note, such a poor correlation between antioxidant activity and total flavonoid content has been reported before 39, 40.
The highest total phenolic content and the highest antioxidant activity among the fifteen plants investigated was found for the M. emarginata fruit extract. This finding is in agreement with previous reports mentioning that an aqueous extract of M. emarginata fruit displayed very potent in vitro antioxidant activity 41, 42 and that its antioxidant activity correlated positively with its total phenolic content 43, 44. Furthermore, a methanol extract of M. emarginata fruit had the highest antioxidant activity among ten other underutilized fruits of Andaman Islands (India) 43 and the highest phenolic content among eleven fruits from Ranong Province and local markets in Bangkok (Thailand) 44. The antioxidant activity has been associated with, among others, phenolic compounds such as benzoic acid derivatives, phenylpropanoid derivatives, flavonoids, and anthocyanins, in addition to several carotenoids and an abundant amount of ascorbic acid 42.
The antioxidant activities of the extracts from A. carambola, A. occidentale, and O. bacaba fruit as well as that of H. sabdariffa calyx were in the intermediate to high range and these samples - along with that from C. latifolium leaf - had the second highest total phenolic content when compared to M. emarginata fruit. These observations are in accordance with the strong positive correlation found between total phenolic content and antioxidant activity for A. carambola fruit 45. The current findings are also in agreement with the high antioxidant activity reported for A. carambola, A. occidentale, and O. bacaba fruit as well as H. sabdariffa calyx 45, 46, 47, 48. For the A. carambola sample, this was probably attributable to polyphenolic compounds such as gallic acid, syringic acid, p-coumaric acid, epicatechin, isoquercetin, and procyanidin B2 in addition to ascorbic acid 49; for A. occidentale fruit preparations to proanthocyanidins, flavonoids, anthocyanins, tannins as well as ascorbic acid 50; for O. bacaba fruit to various phenolic compounds including flavonoids and anthocyanins 51; and for (methanol extracts of) H. sabdariffa calyx mainly to flavonoids, anthocyanins, phenylpropanoids, and carotenoids 52.
As mentioned above, the C. latifolium leaf sample also displayed an intermediate to high antioxidant activity and an intermediate total phenolic content in the current study. Unfortunately, to the best of our knowledge, there are no literature data available for comparison with our findings. However, leaf extracts from other Cestrum species such as the purple cestrum C. elegans, the red cestrum C. fasicilatum, the green cestrum C. parqui, and the night-blooming cestrum C. nocturnum elicited, comparably to that of C. latifolium in the current study, notable antioxidant activity 53, 54. Phytochemical analyses revealed that C. elegans, C. fasicilatum, and C. parqui leaves were negative for phenolic compounds but positive for relatively high amounts of flavonoids 54, whereas methanol extracts of various parts of C. nocturnum contained substantial amounts of both flavonoids and phenols and exhibited notable free radical-scavenging properties 53. Thus, the precise involvement of phenolics and flavonoids in the antioxidant activity of the C. latifolium leaf sample remains to be determined.
The extracts from E. oleracea, P. granatum, and S. cumini fruit displayed an intermediate to high antioxidant activity but a low total phenolic content in the current study. These findings are not in accordance with literature data mentioning that these parts of the plants had high antioxidant activity. Indeed, several investigators reported substantial antioxidant activity of, and considerable quantities of phenolics, - particularly anthocyanins - in E. olearacea fruit pulp 55, 56; appreciable antioxidant activity and a relatively high phenolic content of P. granatum juice that included, among others, gallic acid, chlorogenic acid, caffeic acid, ellagic acid, catechin, epicatechin, quercetin and rutin 57, 58; and meaningful antioxidant activity and significant amounts of phenolics, - particularly anthocyanins and tannins - as well as carotenoids and antioxidant vitamins in the fruit of S. cumini59, 60.
The discrepancy between the relatively low total phenolic contents found for the E. oleracea, P. granatum, and S. cumini samples in the current study, and values reported in the literature, could possibly be ascribed, at least in part, to differences in the extraction methods applied. For instance, samples of P. granatum peel, seed, and seed coat displayed much higher antioxidant activities and phenolic contents upon extraction with 0.1 M HCl: ethanol when compared to those extracted with distilled water 61. The inconsistency between the intermediate to high antioxidant activity of the samples and their relatively low total phenolic contents suggests that phenolics were not the only or the major contributors to their antioxidant activity, and that other secondary metabolites might be involved in this activity. Markedly, for E. oleracea fruit pulp, the two major anthocyanins (cyanidin-3-glucoside and cyanidin-3-rutinoside) reportedly contributed for about 10% to its overall antioxidant activity, signifying that unidentified substances were responsible for the largest part of activity 62. Of note, non-phenolic antioxidant secondary metabolites such as volatile oils, carotenoids, polyunsaturated fatty acids, polysaccharides, and vitamins have also been found to be mainly responsible for the antioxidant activities of certain algae 63.
The extracts from R. alpinia fruit and A. vera leaves displayed (very) low antioxidant activity and total phenolic contents in the current study. These findings are partially in line with the relatively low antioxidant activity reported for R. alpinia fruit pulp 64 despite the presence of phenolic compounds, flavonoids, carotenoids, anthocyanins, and vitamins in this part of the plant, some of which are responsible for the yellow color of the pulp and the red-purple color of its peel 64. It is possible that these compounds, similarly to those addressed in the preceding paragraph 61, 62, 63, did not possess major antioxidant activity, but this supposition must be verified in future studies.
The very low antioxidant activity of the A. vera leaf preparation seen in the current study is at variance with studies reporting high antioxidant activity of a leaf extract of the plant 65, 66. This has been ascribed to, among others, flavonoids, tannins, β-carotene, as well as vitamins C and E 65, 66. The dissimilarities between the results from the current study and those mentioned in the literature could be due to the often described variability in biological activity of A. vera samples caused by differences in the state of maturity and genotype; conditions of cultivation, harvest time, climatic factors, and the method for harvesting 44, 67, and/or the method of extraction and the solvent used for extraction 68.
The samples from S. dulcis, A. muricata, L. acutangula, and S. aqueum fruit had the lowest antioxidant activity and total phenolic content. For the preparations of S. dulcis and A. muricata fruit, these findings are in line with previous observations indicating that the ethanol extracts of S. dulcis and S. cumini fruit indeed displayed a relatively low DPPH radical scavenging activity and total phenolic content in a study with eleven cheap Bangladeshi fruits 69. Furthermore, although A. muricata fruit contains phenolic compounds, flavonoids, ascorbic acid, carotenoids, as well as acetogenins with antioxidant activity 70, an ethanolic extract of Sri Lankan A. muricata fruit pulp displayed only a moderate antioxidant activity and total phenolic content when compared to, for instance, the Italian A. cherimola as well as pomegranate and mango fruits 71.
The current findings with the L. acutangula and S. dulcis samples could tentatively be explained by the dependence of their antioxidant activity and phenolic content on the polarity of the solvent used, extractions with more polar solvents yielding less activity and phenolics 72, 73. In this respect, a methanol extract of L. acutangula fruit and several derived apolar fractions displayed appreciable antioxidant activities (which, however, did not correlate with phenolic and flavonoid contents) while the residual aqueous fraction did not 73. And S. aqueum fruit reportedly displayed notable antioxidant activity 43 and represented a rich source of phenolics and flavonoids 74 including anthocyanidines 75 but yielded less antioxidant activity and phenolic compounds when extracted with distilled water instead of methanol 43.
Conclusions
The results from the current study showed that preparations from M. emarginata, A. carambola, A. occidentale, and O. bacaba fruit as well as C. latifolium leaf and H. sabdariffa calyx displayed relatively high antioxidant activity that correlated well with a high phenolic content. These observations may qualify these plants as ‘genuine’ adaptogens and may help account for some of their claimed medicinal properties 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. Importantly, these plants may represent novel natural sources of antioxidants and bioactive health-promoting phytochemicals. The samples from S. dulcis, A. muricata, E. oleracea, L. acutangula, P. granatum, S. aqueum, S. cumini, and R. alpinia fruit as well as that from A. vera leaf displayed relatively low antioxidant activities and phenolic contents. This suggests that these plants should not be considered ‘genuine’ adoptogens. However, the possibility exists that their adoptogenic qualities are attributable to compounds other than phenolic antioxidants such as carotenoids and/or vitamins C and E. It is also possible that the method of extraction - using distilled water instead of, for instance, methanol - was insufficiently efficient to produce phenolic antioxidants. Studies to assess these possibilities in our laboratories are currently in preparation.
Funding
This study was partially supported by the Suriname Conservation Foundation (project number SCF. 2012.005).
References
- 1.Turrens J F. (2003) Mitochondrial formation of reactive oxygen species. , J Physiol 552(2), 335-344.
- 2.Phaniendra A, Jestadi D B, Periyasamy L. (2015) Free radicals: properties, sources, targets, and their implication in various diseases. , Indian J Clin Biochem 30(1), 11-26.
- 3.Habibi E, Shokrzadeh M, Ahmadi A, Chabra A, Naghshvar F. (2018) Pulmonoprotective action ofZatariamultifloraethanolic extract on cyclophosphamide-induced oxidative lung toxicity in mice.Chin. , J Integr Med 1-3.
- 4.Cappellini M D, Fiorelli G. (2008) Glucose-6-phosphate dehydrogenase deficiency. , Lancet 371(9606), 64-74.
- 5.Azadbakht M, Sj Hosseinimehr, Shokrzadeh M, Habibi E, Ahmadi A. (2011) lotusL. fruit extract protects G6PD-deficient erythrocytes from hemolytic injuryin vitroandin vivo: prevention of favism disorder. Eur Rev Med Pharmacol Sci. 15(11), 1270-1281.
- 6.Ray P D, Huang B W, Tsuji Y. (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. , Cell Signal 24(5), 981-990.
- 7.Zhang J, Wang X, Vikash V, Ye Q, Wu D. (2016) ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 1-18.
- 8.Farber J L. (1994) Mechanisms of cell injury by activated oxygen species. , Environ Health Perspect. 102 (Suppl 10, 17-24.
- 9.He L, He T, Farrar S, Ji L, Liu T. (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. , Cell Physiol Biochem 44(2), 532-553.
- 10.Valko M, Leibfritz D, Moncol J, Cronin M T, Mazur M. (2007) Free radicals and antioxidants in normal physiological functions and human disease. , Int J Biochem Cell Biol 39(1), 44-84.
- 11.Shokrzadeh M, Naghshvar F, Ahmadi A, Chabra A, Jeivad F. (2014) The potential ameliorative effects of melatonin against cyclophosphamide-induced DNA damage in murine bone marrow cells. , Eur Rev Med Pharmacol Sci 18(5), 605-611.
- 12.Cabello-Verrugio C, Simon F, Trollet C, Santibañez J F. (2017) Oxidative stress in disease and aging: mechanisms and therapies 2016. Oxid Med Cell Longev. 1-2.
- 13.Balsano C, Alisi A. (2009) Antioxidant effects of natural bioactive compounds. , Curr Pharm Des 15(26), 3063-3073.
- 14.Aune D, Keum N, Giovannucci E, Fadnes L T, Boffetta P. (2018) Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. , Am J Clin Nutr 108(5), 1069-1091.
- 15.Jayedi A, Rashidy-Pour A, Parohan M, Zargar M S, Shab-Bidar S. (2018) Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective observational studies. , Adv Nutr 9(6), 701-716.
- 16.Minatel I O, Borges C V, Ferreira M I, HAG Gomez, CYO Chen. (2017) Phenolic compounds: functional properties, impact of processing and bioavailability (Chapter 1). In:. Soto-Hernandez M, Palma-Tenango M, del Rosario Garcia-Mateos M (eds.):Phenolic compounds - biological activity. London: IntechOpen 1-24.
- 17.Caleja C, Ribeiro A, Barreiro M F, ICFR Ferreira. (2017) Phenolic compounds as nutraceuticals or functional food ingredients. Curr Pharm Des. 23(19), 2787-2806.
- 18.Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. , Medicines (Basel) 5(3), 1-16.
- 19.Panossian A. (2017) Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. , Ann N Y Acad Sci 1401(1), 49-64.
- 20.DRA Mans, Ganga D, Kartopawiro J. (2017) Meeting of the minds: traditional herbal medicine. in multiethnic Suriname (Chapter 6). In: El-Shemy, HA (ed.):Aromatic and medicinal plants - Back to nature. Rijeka: InTech 111-132.
- 21.May A F. (1982) Sranan oso dresi. Surinaams kruidenboek [Surinamese folk medicine. A collection of Surinamese medicinal herbs]. Paramaribo: De Walburg Pers.
- 22.Titjari. (1985) Famiri-encyclopedia foe da natoera dresi-fasi. Gezinskruidenboek van de natuurgeneeswijzen. Natuurgeneeswijzen uit het zonnige Suriname [Encyclopedia of plantbased forms of treatment. Folk medicines from sunny Suriname]. , Amsterdam: Sangrafoe
- 23.Tjong Ayong G. (1989) Het gebruik van medicinale planten door de Javanen in Suriname [The use of medicinal plants by the Javanese in Suriname]. Paramaribo: Teachers College.
- 24.Heyde H. (1992) Geneesplanten in Surinaamse [Surinamese medicinal plants]. , Paramaribo: Westfort
- 25.Sedoc N O. (1992) Afrosurinaamse natuurgeneeswijzen: bevattende meer dan tweehonderd meest gebruikelijke geneeskrachtige kruiden [Afro-Surinamese natural remedies: over two hundred commonly used medicinal herbs].Paramaribo:VacoPress.
- 26.UPD Raghoenandan. University of Suriname (1994) Etnobotanisch onderzoek bij de Hindoestaanse bevolkingsgroep in Suriname [An ethnobotanical investigation among Hindustanis in Suriname]. Paramaribo: Anton de Kom.
- 27.Van Andel TR, Behari-Ramdas J, Havinga R M, Groenendijk S. (2007) The medicinal plant trade in Suriname. , Ethnobot Res Appl 5, 351-373.
- 28.Slagveer J L. (2009) Sranan oso dresi. Geneeskracht uit Suriname [Surinamese herbal medicines. Healing power from Suriname]. , Paramaribo:
- 29.Van Andel TR, Ruysschaert S. (2011) Medicinal and ritual plants of Suriname.Amsterdam:KITPublishers.
- 30.DRA Mans, Grant A. (2017) A thing of beauty is a joy forever”. Plants and plant-based preparations for facial care in Suriname. , Clin Med Invest 2(4), 1-16.
- 32.IFF Benzie, Szeto Y T. (1999) Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. , J Agric Food Chem 47(2), 633-636.
- 33.Brand-Williams W, Cuvelier M E, Berset C. (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 28(1), 25-30.
- 34.Singleton V L, Orthofer R, Lamuela-Raventos R M. (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. , Methods Enzymol 299, 152-178.
- 35.Chang C C, Yang M H, Wen H M, Chern J C. (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. , J Food Drug Anal 10(3), 178-182.
- 36.Shokrzadeh M, Ahmadi A, Ramezaninejhad S, Shadboorestan A. (2015) Hesperidin, a citrus bioflavonoid, ameliorates genotoxicity-induced by diazinon in human blood lymphocytes. , Drug Res 65(2), 57-60.
- 37.Cai Y, Luo Q, Sun M, Corke H. (2004) Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. , Life Sci 74(17), 2157-2184.
- 38.Li M H, Chen J M. (2008) Investigation of Danshen and related medicinal plants in China. , J Ethnopharmacol 120(3), 419-426.
- 39.Ghasemi Pirbalouti A, Siahpoosh A, Setayesh M, Craker L. (2014) Antioxidant activity, total phenolic and flavonoid contents of some medicinal and aromatic plants used as herbal teas and condiments in Iran. , J Med Food 17(10), 1151-1157.
- 40.Chaudhari G M, Mahajan R T. (2015) Total flavonoid content correlated with antioxidant activity to lower extent as compared to phenolic content. , Int J Pharm Sci Rev Res 30(1), 105-111.
- 41.Wang L, Li F, He C, Dong Y, Wang Q. (2015) Antioxidant activity and melanogenesis inhibitory effect of acerola fruit (Malpighia glabraL.) aqueous extract and its safe use in cosmetics. , Asian J Chem 27(3), 957-960.
- 42.Belwal T, Devkota H P, Hassan H A, Ahluwalia S, Ramadan M F. (2018) Phytopharmacology of acerola (Malpighiaspp.) and its potential as functional food. Trends Food Sci Technol. 74(7), 99-106.
- 43.Singh D R, Singh S, Salim K M, Srivastava R C. (2012) Estimation of phytochemicals and antioxidant activity of underutilized fruits of Andaman Islands (India). , Int J Food Sci Nutr 63(4), 446-452.
- 44.Anantachoke N, Lomarat P, Praserttirachai W, Khammanit R, Mangmool S. (2016) Thai fruits exhibit antioxidant activity and induction of antioxidant enzymes in HEK-293 cells. Evid Based Complement Alternat Med.eCAM. 1-14.
- 45.Asna A N, Noriham A. (2014) Antioxidant activity and bioactive components of Oxalidaceae fruit extracts. , Malaysian J Anal Sci 18(1), 116-126.
- 46.Ramakrishna B V, Jayaprakasha G K, Jena B S, Singh R. (2008) Antioxidant activities of roselle (Hibiscus sabdariffa) calyces and fruit extracts. , J Food Sci Technol 45(3), 223-227.
- 47.Barbosa-Filho V M, Waczuk E P, Kamdem J P, Abolaji A O, Lacerda S R. (2014) Phytochemical constituents, antioxidant activity, cytotoxicity and osmotic fragility effects of caju (Anacardiummicrocarpum). Ind Crops Prod. 55, 280-288.
- 48.Dos Santos MFG, RVS Mamede, MSM Rufino, ESB Brito, Alves R E. (2015) Amazonian native palm fruits as sources of antioxidant bioactive compounds. Antioxidants (Basel). 4(3), 591-602.
- 49.Pang D, You L, Li T, Zhou L, Sun-Waterhouse D. (2016) Phenolic profiles and chemical- or cell-based antioxidant activities of four star fruit (Averrhoacarambola) cultivars. , RSC Advances 6(93), 90646-90653.
- 50.MTS Trevisan, Pfundstein B, Haubner R, Würtele G, Spiegelhalder B. (2006) Characterization of alkyl phenols in cashew (Anacardiumoccidentale) products and assay of their antioxidant capacity. Food Chem Toxicol. 44(2), 188-197.
- 51.AFD Finco, Kammerer D R, Carle R, Tseng W H, Böser S. (2012) Antioxidant activity and characterization of phenolic compounds from bacaba (OenocarpusbacabaMart.) fruit by HPLC-DAD-MS(n). , J Agric Food Chem 60(31), 7665-7673.
- 52.Anokwuru C P, Esiaba I, Ajbaye O, Adesuyi A O. (2011) Polyphenolic content and antioxidant activity ofHibiscus sabdariffacalyx. , Res J Med Plants 5(5), 557-566.
- 53.Rashed K. (2013) Investigation of antioxidant activity fromCestrumnocturnumL. stems and phytochemical content. Rev Progr. 1(5), 1-6.
- 54.Chikkaswamy B K. (2015) Anti-oxidant potential, antimicrobial activities and phytochemical screening, in three species ofCestrum. , Int J Adv Res IT Engineering 4(3), 1-10.
- 55.Agawa S, Sakakibara H, Iwata R, Shimoi K, Hergesheimer A. (2011) Anthocyanins in mesocarp/epicarp and endocarp of fresh açai (Euterpe oleraceaMart.) and their antioxidant activities and bioavailability. Food Sci Technol Res. 17(4), 3270-334.
- 56.Garzón G A, Narváez-Cuenca C E, Vincken J P, Gruppen H. (2017) Polyphenolic composition and antioxidant activity of açai (Euterpe oleraceaMart.) from Colombia. Food Chem. 217, 364-372.
- 57.Anahita A, Asmah R, Fauziah O. (2015) Evaluation of total phenolic content, total antioxidant activity, and antioxidant ascorbic acid composition of pomegranate seed and juice. , General Med 3(1), 1-4.
- 58.Hmid I, Elothmani D, Hanine H, Oukabli A, Mehinagic E. (2017) Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (PunicagranatumL.) cultivars grown in Morocco. , Arab J Chem 10(2), 2675-2684.
- 59.Banerjee A, Dasgupta N, B De. (2005) vitrostudy of antioxidant activity ofSyzygiumcuminifruit. , Food Chem 90(4), 727-733.
- 60.Singh J, Shukla R K, Walia S. (2013) Sugar profile, total phenolic and antioxidant potential of anthocyanins richSyzygiumcuminifruit. , Nat Prod Indian J 9(9), 350-354.
- 61.Pengkumsri N, Kaewdoo K, Leeprechanon W, Sivamaruthi B S. (2019) Influence of extraction methods on total phenolic content and antioxidant properties of some of the commonly used plants in Thailand. , Pakistan J Biol Sci 22(3), 117-126.
- 62.Lichtenthäler R, Rodrigues R B, Maia J G, Papagiannopoulos M, Fabricius H. (2005) Total oxidant scavenging capacities ofEuterpe oleraceaMart. (açaí) fruits. , Int J Food Sci Nutr 56(1), 53-64.
- 63.Chen F, Li H B, RNS Wong, Ji B, Jiang Y. (2005) Isolation and purification of the bioactive carotenoid zeaxanthin from the microalgaMicrocystis aeruginosaby high-speed countercurrent chromatography. , J Chromatogr A 1064(2), 183-186.
- 64.MLL Guevara, CEO Velasco, Caranza P H, LEUC Cortes, JJL Guevara. (2018) Composition, physico-chemical properties and antioxidant capacity ofRenealmiaalpinia(Rottb.) Maas fruit. Rev FCA UNCUYO. 50(2), 377-385.
- 65.Sazhina N N, Lapshin P V, Zagoskina N V, Misin V M. (2016) Comparative study of antioxidant properties of extracts of variousAloespecies. , Russ J Bioorg Chem 42, 735-740.
- 66.LLO Rodrigues, de Oliveira ACL, Tabrez S, Shakil S, Khan M I. (2018) Mutagenic, antioxidant and wound healing properties ofAloe vera. , J Ethnopharmacol 227, 191-197.
- 67.Kumar S, Yadav A, Yadav M, Yadav J P. (2017) Effect of climate change on phytochemical diversity, total phenolic content andin vitroantioxidant activity ofAloe vera(L.) Burm.f. , BMC Res Notes 10(60), 1-12.
- 68.Sultana B, Anwar F, Ashraf M. (2009) Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. , Molecules 14(6), 2167-2180.
- 69.Hossain S J, Tsujiyama I, Takasugi M, Islam A, Biswas R S. (2008) Total phenolic content, antioxidative, anti-amylase, anti-glucosidase, and antihistamine release activities of Bangladeshi fruits. Food Sci Technol Res. 14(3), 261-268.
- 70.Adefegha S A, Oyeleye S I, Oboh G. (2015) Distribution of phenolic contents, antidiabetic potentials, antihypertensive properties, and antioxidative effects of soursop (AnnonamuricataL.) fruit partsin vitro. , Biochem Res Int 1-8.
- 71.SMPC Padmini, Samarasekera R, DKNG Pushpakumara. (2015) Antioxidant capacity and total phenol content of Sri LankanAnnonamuricataL. Trop Agricult Res. 25(2), 252-260.
- 72.SMA Islam, Ahmed K T, Manik M K, Wahid M A, CSI Kamal. (2013) A comparative study of the antioxidant, antimicrobial, cytotoxic and thrombolytic potential of the fruits and leaves ofSpondiasdulcis. , Asian Pac J Trop Biomed 3(9), 682-691.
- 73.Suryanti V, Marliyana S D, Wulandari T. (2015) Antioxidant activity, total phenolics and flavonoids contents ofLuffaacutangula(L.) Roxb fruit. , J Chem Pharm Res 7(1), 220-226.
Cited by (12)
This article has been cited by 12 scholarly works according to:
Citing Articles:
Biochemistry (2023) OpenAlex
(2023) Crossref
Biochemistry (2023) OpenAlex
(2023) Crossref
World Journal of Biology Pharmacy and Health Sciences (2023) OpenAlex
D. R. Mans, P. Friperson, J. Pawirodihardjo, M. Djotaroeno - World Journal of Biology Pharmacy and Health Sciences (2023) Semantic Scholar
GSC Biological and Pharmaceutical Sciences (2022) OpenAlex
Research Journal of Medicinal Plant (2021) OpenAlex
Research Journal of Medicinal Plants (2021) Crossref
International and cultural psychology/International and cultural psychology series (2021) OpenAlex
M. Sanches, T. Graafsma, Glenn Leckie - International and Cultural Psychology (2021) Semantic Scholar

