Different Effects of Ethanol and Activation of TRPM8 ION Channel on Metabolic Response to Cold
Abstract
The possible interrelation of ethanol and the membrane protein molecules such as TRP ion channels in the whole living organism has not been studied. In the present research we study the influence of ethanol (50%) and agonist of TRPM8 ion channel L-menthol (1% in 50% ethanol) application to abdominal skin on the thermoregulatory response to cooling in rats. We used two types of cooling with the different rates of skin temperature decrease - 0.1 °C/sec for rapid and 0.005°C/s for slow cooling.
It was shown, that the effects of ethanol and activation of the cold-sensitive TRPM8 ion channel are mainly directed at different components of thermoregulatory metabolic response to cold. Menthol, as an agonist of the TRPM8 ion channel, besides the constrictor vascular response stimulates predominantly the emergency first phase of metabolic response which appears only at rapid cooling without any effect on the second phase of metabolic response to cooling. Ethanol inhibits the most powerful second phase of metabolic response to cold which is manifested at decreased deep body temperature and is associated with the development of not only non-shivering but also shivering thermogenesis. Effect of ethanol is accompanied by the acceleration of the deep body temperature fall. Ethanol does not prevent the effect of menthol on thermoregulatory blood vessel and emergency phase of metabolic response, and the activation of the cold-sensitive TRPM8 ion channel by menthol has no obvious influence on the effects of ethanol – inhibition being the most powerful thermogenic component of the metabolic response to cold.
Author Contributions
Academic Editor: S Bhupendra Kaphalia, University of Texas Medical
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Tamara V. Kozyreva, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Ethanol while being introduced into the organism mainly through ingestion or skin application affects many functional systems. The basis for the influence of ethanol on the body functioning is its direct effect on the cell metabolic processes. Ethyl alcohol affects the cell membrane by separating fatty acid chains of phospholipids and changing membrane fluidity; it also alters the permeability of volt- and ligand-gated ion channels and directly alters the electrical potentials of neurolemma.
Ambient temperature is one of the major factors affecting the living organism. The start thermal afferent information under the external cooling primarily is given by the peripheral skin thermo-afferents. In recent years there have been numerous studies concerning the cellular and molecular mechanisms of cold sensitivity. It is believed that thermosensitive TRP channels are the basis for temperature sensation1. Perception of cold in the physiologically relevant temperature range is performed with participation of the cold-sensitive ion channel TRPM8, which is activated by cooling in the range of 28-8ºC. Menthol is one of known agonists of the TRPM8 ion channel. The expression of the gene of the TRPM8 ion channel has been proved on the endings of sensory neurons, spinal ganglia and brain structures2, 3, 4. Free endings of sensory neurons are the peripheral thermoreceptors (thermo-afferents). Thus the perception of cold and the afferent signal depend on the activity of cold-sensitive ion channels, which increase its permeability in response to the decrease in temperature1, 5.
Afferent signal determines the character and sequence of various physiological responses initiation to some effect. Temperature thresholds for cold-defense responses, aimed at maintaining temperature homeostasis, characterize thermal afferent signal at external cold exposure. The study of the thermal afferent signal role that is formed under the effect of pharmacological modulation of the skin thermoreceptor activity is of particular interest. We have previously shown that pharmacological activation of cold-sensitive TRPM8 ion channels leads to the shift in temperature thresholds of responses to cooling6. What are the effects of ethanol on the temperature thresholds as well as the structure of thermoregulatory response of the whole organism to cold exposure is not currently known. Ethanol, being able quickly to penetrate the lipid-rich membrane, and causing the conformation of the protein molecules, can alter the function of ion channels. Currently, there are data that ethanol interacts with the molecules such as TRP ion channels7, 8, 9. So, it was shown in vitro on HEK293T cells that ethanol inhibits cold-sensitive TRPM8, but activates the warm-sensitive TRPV1 ion channel7. The possible interaction of ethanol and the TRPM8 ion channel in the whole living organism is not known.
In the present research we try to find the answer to following questions1. How the application of ethanol to skin (the area of thermosensitive afferents concentration) affects temperature thresholds and thermoregulatory blood vessels and metabolic response to rapid and slow cooling?2. What is the interrelation of the ethanol effect and effect of the TRPM8 ion channel activation in the formation of cold-defense metabolic responses?
Methods
Animals
Male Wistar rats weighing 180-220 g were used. The animals were housed at ambient temperature 22-24°C, natural dark-light cycle with free access to water and food. The experimental procedures were in compliance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010, its correction of 20 December 2013 (2014/63/EU) and approved by the ethic committee of the Institute of Physiology and Fundamental Medicine. All procedures including the thermocouple and muscle electrodes fixation, application of drugs and cooling were done under anesthesia (Nembutal, 40 mg/kg) to exclude the emotional component and moving in the animals.
Cooling
Experiments were performed at the room temperature of 20-22°C and normal humidity 40-50%. The initial precooling temperature of the animal was maintained by the temperature controlled panel so that the rectal temperature was 38.4±0.13°C; the abdomen skin temperature was 38.4±0.11°C. Skin free of hair in the abdominal area of 25 cm2 was cooled using thermostat. Removal of hair was carried out with scissors without any chemical drugs and painful irritation.
The experimental models of rapid and slow cooling were used. According to the previous neurophysiologic data recording the firing rate of the skin cold-sensitive nerve endings 10, 11, if the rate of skin temperature fall is lower than 0.01-0.02 °C/sec there is no dynamic activity of thermosensitive afferents. So we chose the rate of cooling 0.1 °C/sec for rapid and of 0.005⁰C/s for slow cooling being fully aware that at slow cooling there is only static activity of the skin cold-sensitive fibers (nerve firing rate in accordance with temperature) and at rapid cooling - not only static, but also dynamic activity (the starting short term increase in firing rate) of the skin cold-sensitive fibers. Cooling (rapid or slow) was performed until a decrease of rectal temperature by 3-4°C was reached.
Ethanol and Menthol Application
In thermoneutral conditions before cold exposure 50% ethanol or 1% menthol (L–Menthol, Sigma) in 50% ethanol was applied to skin, where thermoreceptors are concentrated. Drugs were applied for 20 minutes in the area of abdomen (25 cm2) where the cold stimulus was further applied. For this purpose, 1 ml of drug was evenly spread over filter paper of the corresponding size and applied to the skin. After 20 minutes the filter paper was removed. Temperature of the applied drugs was 37-38°C. The distillated water (Aqua purificata) was applied to control animals.
Thermoregulatory Response
The following thermoregulatory parameters were continuously measured throughout the experiment: (1) temperature at a site remote from the cooled and isolated from the environmental and cooling effects, the auricular floor skin; this allowed us to judge how skin vessels respond to the application of drugs and cooling; (2) rectal temperature to measure core temperature and determine the threshold rectal temperature for the cold defense responses; (3) intracutaneous temperature of the cooled abdominal surface to control cooling rate; (4) total oxygen consumption and carbon dioxide release to estimate metabolic response (thermogenesis); (5) electrical activity of neck muscles to estimate cold defense muscle activity (shivering). The following changes during cooling were accepted as threshold values: 0.1 °C for temperatures, 1 ml/min kg for oxygen consumption and carbon dioxide release, 1 μV for electrical muscle activity. For measuring oxygen consumption and dioxide release mask and Gas Concentration Measurement Modules (O2100C and CO2100C, Biopac) were used, the collected gas was injected into the analyzer via Gas Sampling Interface Kit with NAFION® dryer. The value of CO2/O2 was calculated to estimate the changes in respiratory coefficient. All the parameters were recorded using computer IBM PC, applying the “Biopac” system. To determine the effect of drugs, the parameters of the cold defense responses were evaluated during cooling in rats - without and with preceding administration of drugs.
Design of Experiment
Animals were anesthetized, and then all the sensors were fixed (for registration of temperatures, muscle activity, oxygen consumption and carbon dioxide release). Within 5-10 minutes the initial parameters were recorded, then at continuous recording of parameters the application of drugs or distillate water was carried out during 20 min, which was followed by cold exposure. This allowed us to identify the effect of drug in thermoneutral conditions, and in cold. In our previous studies we tested the effect of distillate water and found no effect 12. Every animal was cooled only once. There were 6 experimental groups of rats: (1) rapid and (2) slow cooling with distillate water application (controls, n=13 and n=12); (3) rapid and (4) slow cooling on the background of 50% ethanol application (n=9 and n=8); (5) rapid and (6) slow cooling on the background of 1% menthol solution in 50% ethanol application (n=10 and n=10).
Statistical Analysis
The data are presented as means ± S.E.M. and were treated for significance by the Student’s “t” test with the program “Statistica”.
Results
In thermoneutral conditions application to the skin of 50% ethanol or 1% solution of menthol in 50% ethanol had no effect on the registered parameters (Table 1).
Table 1. Effect of application to the skin of 50% ethanol or 1% menthol in 50% ethanol on the parameters of temperature homeostasis in thermoneutral conditions without cold load.| Parameters | Control | Ethanol 50% | Menthol 1% in 50% ethanol |
| Abdomen skin temperature (°С) | 38,4±0,11 | 38,5±0,10 | 38,5±0,07 |
| Ear skin temperature (°С) | 32,9±0,36 | 32,8±0,35 | 32,5±0,40 |
| Rectal temperature (°С) | 38,4±0,13 | 38,4±0,12 | 38,4±0,12 |
| Electrical muscle activity (µV) | 3,9±0,53 | 3,9±0,62 | 4,3±0,81 |
| Oxygen consumption (ml/min kg) | 23,7±0,69 | 23,4±0,72 | 24,1±0,84 |
| Carbon dioxide release (ml/min kg) | 17,8±0,54 | 17,4±0,59 | 17,9±0,63 |
| Respiratory coefficient (units) | 0.75±0,016 | 0.74±0,016 | 0.74±0,012 |
There is a definite sequence of thermoregulatory responses characteristic to rapid and slow deep cooling6. At rapid cooling (Figure 1, left panel), to begin with, when only skin temperature decreases, the first phase of metabolic response characterized by rising respiratory coefficient develops, i.e. increased utilization of carbohydrates. Then, with greater cooling of the skin, but also without change in core temperature, a constrictor response of the skin blood vessels has been initiated. The second phase of metabolic response characterized by a decrease in the respiratory coefficient (increase of lipid metabolism) enfolds when not only skin, but also deep body temperature falls, this is associated with an increase in thermogenic muscle activity (shivering). At slow cooling (Figure 2, left panel), the first phase of metabolic response associated with the dynamic activity of skin thermoreceptors is absent. Cold-defense response includes the constriction of skin blood vessels and metabolic response, the character of which is similar to the second phase of the metabolic response at rapid cooling.
Figure 1.Development of thermoregulatory responses to control rapid cooling (without any drugs) and rapid cooling on the background of 50% ethanol application to skin. The units for temperatures - °C, for O2 consumption and CO2 release – ml/min*kg, for electrical muscle activity – mV, for respiratory coefficient – units.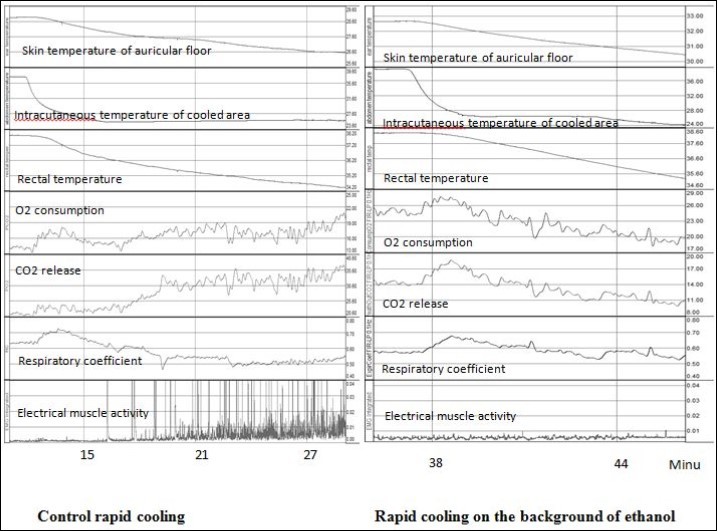
Rapid Cooling
At rapid cooling the application of 50% ethanol has not affected the parameters of skin blood vessel response, whereas the application of menthol solution in ethanol accelerated the initiation of constrictor response, which occurred at a higher skin temperature, i.e. the threshold decrease in skin temperature for this response was lower (Table 2).
Table 2. Effects of 50% ethanol and 1% solution of menthol in 50% ethanol on the skin blood vessel response to rapid cooling| Parameters | Control | Ethanol 50% | Menthol 1% in 50% ethanol |
| Latency, sec | 44,0±4,90 | 47,0±7,06 | 35,4±2,47* |
| Threshold skin temperature, °С | 33,0±0,53 | 33,1±0,67 | 35,7±0,26* |
| Threshold decrease in skin temperature, °С | 4,7±0,46 | 5,2±0,67 | 2,6±0,23* |
| Threshold rectal temperature, °С | 38,1±0,11 | 38,4±0,13 | 38,4±0,20 |
| Threshold decrease in rectal temperature, °С | 0,08± 0,04 | 0,02±0,009 | 0,02±0,007 |
| Maximum decrease in the ear skin temperature under the cooling, °С | 2,9±0,33 | 2,5±0,32 | 3,4±0,54 |
Application of 50% ethanol had no influence on the temperature thresholds and the maximum value of oxygen consumption during the first phase of metabolic response at rapid cooling (Table 3). However, on the background of ethanol it was not observed the significant increase in the respiratory coefficient (Table 3, second column), whereas in the control group without any drugs there was an increase in the respiratory coefficient (P<0.05) in the first phase of the metabolic response to cold, see also6. On the background of menthol solution in ethanol at rapid cooling the decrease in latency and the skin temperature threshold for the first phase of metabolic response to rapid cooling was observed. However, similar to the background of pure ethanol, there was no characteristic for the first phase increase in the respiratory coefficient (Table 3, third column).
Table 3. Effects of 50% ethanol and 1% solution of menthol in 50% ethanol on the parameters in the first phase of metabolic response to rapid cooling| Parameters | Control | Ethanol 50% | Menthol 1% in 50% ethanol |
| Latency, sec | 39.3±5.29 | 30.3±4.71 | 19.1±3.54** |
| Threshold skin temperature, °С | 34.3±0.45 | 34.4±1.02 | 37.0±0.26** |
| Threshold decrease in skin temperature, °С | 3.4±0.41 | 4.0±1.00 | 1.3±0.22*** |
| Threshold rectal temperature, °С | 38,1±0,07 | 38,3±0,17 | 38,4±0,21 |
| Maximum value of respiratory coefficient (units) | 0.80±0,021 | 0.72±0.036* | 0.73±0.017 |
After the application ethanol or solution of menthol in ethanol the second phase of the metabolic response to rapid cooling was totally absent, i.e. increase in oxygen consumption and electrical muscle activity were not observed at deep rapid cooling on the background of both ethanol and menthol in ethanol (Figure 1, right panel).
Slow Cooling
A preliminary application of ethanol or menthol solution in ethanol had no effect on the parameters of the skin blood vessel response to slow cooling (Table 4).
Table 4. Development of the skin blood vessel response to slow cooling without drugs and on the background of 50% ethanol and 1% solution of menthol in 50% ethanol.| Parameters | Control | Ethanol 50% | Menthol 1% in 50% ethanol |
| Latency, sec | 170,9±50,34 | 91.3±16.32 | 118,5±22,25 |
| Threshold skin temperature, °С | 37,2±0,39 | 37.5±0.34 | 36,9±0,28 |
| Threshold decrease in skin temperature, °С | 1,4±0,41 | 1.0±0.15 | 1,4±0,22 |
| Threshold rectal temperature, °С | 38,0±0,15 | 38.5±0.28 | 38,5±0,16 |
| Threshold decrease in rectal temperature, °С | 0,12±0,022 | 0,04±0,019 | 0,05±0,016 |
| Maximum decrease in the ear skin temperature under the cooling, °С | 2.2±0.19 | 2.5±0.23 | 2,6±0,26 |
The metabolic response to slow cooling after the application of ethanol was completely absent and there was no increase in oxygen consumption and electrical muscle activity (Figure 2, right panel). In addition, it was observed even decrease in the total oxygen consumption on the background of ethanol during slow cooling by 14% (P<0.001) and during rapid cooling by 25% (P<0.001) in comparison with the values before cooling.
Figure 2.Development of thermoregulatory responses to control slow cooling (without any drugs) and slow cooling on the background of 50% ethanol application to skin. The units for temperatures - °C, for O2 consumption and CO2 release – ml/min*kg, for electrical muscle activity – mV, for respiratory coefficient – units.
After application of menthol solution in ethanol in 7 animals out of 10 the metabolic response to slow cooling did not develop.
Since ethanol inhibits the most powerful phase of metabolic response to cold which appears at deep body cooling and is associated with the development of shivering (this is the second phase at rapid cooling and the only phase at slow cooling) thermogenesis is decreased and the rate the body temperature fall becomes faster (Table 5). In case of addition of menthol, the fall of rectal temperature becomes slower at rapid cooling (Table 5).
Table 5. Rate of the deep body temperature decrease at cooling in control and on the background of application to skin of ethanol or menthol solution in ethanol.Discussion
The obtained results prove that thermoregulatory response to cooling are largely modified by the preliminary application of the ethanol to skin. It can be assumed that the effect of ethanol in our experiments was caused mainly through the peripheral skin afferents. Previously we have shown that the modulation of the peripheral thermal inputs by various biologically active substances results in changes of the thermal thresholds and other parameters of thermoregulatory responses13, 14, 15.
As it was mentioned above, thermoregulatory responses to rapid and slow cooling are characterized by different components. In current experiments, the application of ethanol and solution of menthol in ethanol differently affects thermoregulatory metabolic response depending on the cooling rate.
At rapid cooling, ethanol has no effect on the first phase of the metabolic response, which takes place only at rapid changes of the skin temperature, i.e. in the presence of the dynamic activity of the skin cold thermoreceptors. This suggests that ethanol does not influence the processes caused by the dynamic activity of the skin thermoreceptors. It should also be noted, that these responses are initiated when only skin temperature decreases before the fall of deep body temperature 6.
On the contrary, according the above mentioned data, ethanol has clear inhibitory effect on the component of the metabolic response to rapid cooling, which normally develops when the deep body temperature falls. Thus, under the influence of ethanol, the second phase of the metabolic response to rapid cooling and shivering thermogenesis which accompanies this phase does not develop and is completely absent. At slow cooling, ethanol similar rapid cooling supresses the metabolic response and shivering does not develop. Inhibition in metabolic response to cold exposure is accompanied by the acceleration of the deep body temperature fall. Thus, at cooling with different rates, ethanol inhibits the most powerful thermogenic component of the metabolic response to cold which develops when the deep body temperature decreases, and has no influence on the earlier started responses which are initiated without considerable decrease in deep body temperature, but only at the rapid decrease in skin temperature. Moreover, on the background of ethanol, instead of increasing thermogenic metabolism during cooling, a significant decrease in oxygen consumption is observed. A number of researchers have also noted the hypothermic effect of the intrinsic administration of ethanol in thermoneutral conditions16,17as well as its inhibitory effect mainly on thermogenesis and not on heat loss18.
Decrease of the main phase of the cold thermogenesis which we observed in our experiments, when applying ethanol to skin, may be due to inhibition of non-shivering thermogenesis in brown adipose tissue, and inhibition of muscle thermogenic activity (shivering). It is known that mitochondrial UCP1 in brown adipose tissue (BAT) is a key molecule for non-shivering thermogenesis. The data on the influence of ethanol on the level of noradrenaline and uncoupling in BAT give evidence for the possibility to change non-shivering thermogenesis by ethanol19. So it was shown, that at room temperature, ethanol did not significantly alter the level of noradrenaline or UCP1 mRNA in BAT, whereas at cold exposure (+4ºC) the noradrenaline level in rats drinking ethanol was significantly lower than in control animals20. Earlier, it was noted that drinking alcohol can delay the onset of shivering and reduce its duration, and this may also result in the decrease of thermogenesis21. The inhibitory effect of ethanol on the contractile activity of skeletal muscles during cooling can be mediated by α-adrenoreceptors, since the important role of these receptors in modulating effects of ethanol is known, as well as their role in muscle contraction22. Previously we also have shown that α-adrenoreceptor antagonist completely abolishes the development of shivering in the cold23.
Recent studies allow us to believe that ethanol can modulate and interrelate with thermosensitive TRP ion channels, which are responsible for the afferent thermal information and the initiation of thermoregulatory responses7, 9. However, presented data as well as our previous results12 have shown that menthol, agonist of the cold-sensitive TRPM8 ion channel, when dissolved in ethanol or saline has the mostly pronounced stimulating effect on the first phase of metabolic response to rapid cooling and the vascular response. So, ethanol does not prevent the effect of menthol. At the same time, the activation of the cold-sensitive TRPM8 ion channel by menthol has no obvious influence on effects of ethanol – inhibition the most powerful thermogenic component of the metabolic response to cold. Consequently, there are no evidences on the inhibiting effect of ethanol on the activity of the TRPM8 ion channel. The available literary data7 on the effect of ethanol on the TRPM8 ion channel were obtained in vitro on the culture of the specific HEK293T cells and may not fully reproduce the processes of the whole living organism.
Thus, the present results prove that modulatory effects of ethanol and activation of the cold-sensitive TRPM8 ion channel are mainly directed at different metabolic components of thermoregulatory response to cold. Menthol, as agonist of the TRPM8 ion channel, predominantly stimulates the emergency first phase of metabolic response which appears only at rapid cooling in the presence of the dynamic activity of the skin cold receptors before the decrease of deep body temperature, while ethanol inhibits the most powerful phase of metabolic response to cold which appears at deep body cooling and is associated with the development of shivering thermogenesis in muscles and non-shivering thermogenesis in brown adipose tissue. It is possible that various effects of ethanol and menthol are due to their impact on different groups of the skin thermal afferents.
Acknowledgements
The work has been supported by Russian Foundation for Basic Research (RFBR) (grant No 14-04-00700a).
References
- 1.Jordt S, McKemy D, Julius D. (2003) Lessons from peppers and peppermint: the molecular logic of thermoregulation. , Current Opinion in Neurobiology 13, 1-6.
- 2.McKemy D, Neuhausser W, Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. , Nature 416, 52-58.
- 3.Reid G, Flonta M. (2001) Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurons. , Neurosci. Lett 297, 171-174.
- 4.I P Voronova, Tuzhikova A A, T V AKozyreva. (2013) Gene expression of thermosensitive TRP ion channels in the rat brain structures: effect of adaptation to cold. , J. Thermal Biology 38(6), 300-304.
- 5.Montell C. (2003) Thermosensation: hot findings make TRPNs very cool. , Curr. Biol. 13,R 476 - R 478.
- 6.T V Kozyreva, V P Kozaruk, E Ya Tkachenko, G M Khramova. (2010) Agonist of TRPM8 channel, menthol, facilitates the initiation of thermoregulatory responses to external cooling. , J. Thermal Biology 35(8), 428-434.
- 7.Benedikt J, Teisinger J, Vyklicky L, Vlachova V. (2007) Ethanol inhibits cold-menthol receptor TRPM8 by modulating its interaction with membrane phosphatidylinositol 4,5-bisphosphate. , J. Neurochem 100(1), 211-224.
- 8.S R Vigna, R A Shahid, R A Liddle. (2014) Ethanol contributes to neurogenic pancreatitis by activation of TRPV1. , FASEB J 28(2), 891-896.
- 9.S E Weil, N J Moore, Waite A, M J Randall, Gunthorp M. (2005) . Conservation of Functional and Pharmacological Properties in the Distantly Related Temperature Sensors TRPV1 and TRPM8. Mol. Pharmacol 68(2), 518-27.
- 10.Davies S, Goldsmith G, Hellon R, Mitchell D. (1983) Facial sensitivity to rates of temperature change: neurophysiological and psychophysical evidence from cats and humans. , J. Physiol 344, 161-175.
- 11.T V Kozyreva. (1992) Modulating of the functional properties of skin thermoreceptors. , Neurophysiology (Kiev) 24(5), 350-357.
- 12.T V Kozyreva, E Ya Tkachenko, L S Eliseeva, V P Kozaruk, E V Polyakova. (2001) A possible mechanism for noradrenaline involvement in the effector responses to cold exposure. , J. Thermal Biology.26(4 – 5), 505-512.
- 13.T V Kozyreva, E Ya Tkachenko, V P Kozaruk. (1999) Thermoregulatory responses to cooling before and after the noradrenaline iontophoresis to skin. , J. Therm. Biol 24, 175-183.
- 14.E Ya Tkachenko, V P Kozaruk, T V Kozyreva. (2006) Effect of Substance P on thermoregulation parameters during different cooling modes. , Bulleten of Experimental Biology and Medicine 141(6), 695-697.
- 15.E Ya Tkachenko, S V Lomakina, T V Kozyreva. (2005) Modulating effect of calcium on the cold defense response formation in normotensive and hypertensive rats. , J. Thermal 30(7), 545-550.
- 16.Kalant H, D Le A. (1983) Effects of ethanol on thermoregulation. , Pharmacol.Ther 23(3), 313-364.
- 17.Lomax P, J G Bajorek, T A Bajorek, Chaffe R R. (1981) Thermoregulatory mechanisms and ethanol hypothermia. , Eur. J. Pharmacol 71(4), 483-487.
- 18.D E Spiers, R M Threatte, M J Fregly. (1984) Response to thermal stress in the rat following acute administration of ethanol. , Pharmacology 28(3), 155-170.
- 19.Huttunen P, Sämpi M, Myllylä R. (1998) Ethanol-induced hypothermia and thermogenesis of brown adipose tissue in the rat. , Alcohol 15(4), 315-318.
- 20.Yoshimoto K, Yasuhara M, Komura S, Misumi Y, Uchiyama Y et al. (2004) Effects of ethanol on the induction of uncoupling protein-1 (UCP1) mRNA in the mouse brown adipose tissue.TohokuJ.Exp.Med.204(1),45-51.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
Neuroscience Letters (2024) OpenAlex
Neuroscience Letters (2024) Crossref
T. Kozyreva, I. V. Orlov, A. R. Boyarskaya, I. Voronova - Neuroscience Letters (2024) Semantic Scholar
Journal of Thermal Biology (2019) OpenAlex
Journal of Thermal Biology (2019) Crossref
T. Kozyreva, V. P. Kozaruk, E. S. Meyta - Journal of Thermal Biology (2019) Semantic Scholar
