Abstract
Background: Heart valves share developmental signaling pathways with cartilage and bone. While calcific aortic valve disease (CAVD) has been associated with valve calcification and stenosis, suggestive of osteogenesis, myxomatous mitral valve disease (MMVD) is characterized by net matrix degradation, exuberant deposition of proteoglycan, and valve regurgitation.
Methods: We determined the presence of cartilage-abundant proteoglycan, aggrecan; cartilage-specific type II collagen; chondrogenic transcription factor, Sox9; and osteogenic transcription factor, Runx2 in human normal and myxomatous mitral valve leaflets by immunohistochemistry.
Results and Conclusions: Myxomatous, but not normal, mitral valves demonstrated sharp focal areas that were abundant in aggrecan, type II collagen, and Sox9. These focal areas co-localized with areas of myxomatous pathologic change on Movat staining. Some cells in these areas had a round and hypertrophic morphology reminiscent of chondrocytes. Runx2 was only weakly present in normal and myxomatous mitral valves. These findings suggest a focal pathologic process in MMVD that mimics chondrogenesis.
Author Contributions
Academic Editor: Jinlei Nie, School of Physical Education and Sports, Macao Polytechnic Institute.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2013 Carla M. R. Lacerda, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The age-adjusted prevalence of valvular heart disease is estimated at 2.5% in the USA, and its prevalence increases sharply after 65 years of age due to the predominance of degenerative etiologies 1, 2. Degenerative valvular heart disease accounts for about 63% of native valvular heart disease. The most common manifestations of degenerative valvular disease are myxomatous mitral valve disease (MMVD) and calcific aortic valve disease (CAVD) 1. While both conditions are considered degenerative, MMVD and CAVD differ significantly in their functional manifestation and pathology. From a hemodynamic standpoint the most typical manifestation of MMVD is valve regurgitation, whereas the most common manifestation of CAVD is valve stenosis. Pathologically MMVD is characterized by gross leaflet thickening with chordae lengthening or rupture, net degradation of the extracellular matrix (ECM), and exuberant deposition of proteoglycan (PG)/glycosaminoglycan (GAG) 3, 4. On the other hand, CAVD is characterized by leaflet immobility, lipid accumulation, progressive leaflet fibrosis and calcification 5. The reasons for these divergent manifestations of valvular degeneration in the mitral and aortic valve are currently not understood.
Research in heart valve development has suggested several commonalities between the pathways regulating this process and chondrogenesis, which in turn might have important roles in adult tissue remodeling. Heart valvulogenesis appears to be highly conserved among vertebrates and is governed by developmental regulatory pathways consisting of receptor-based signaling, transcription factors, and downstream structural genes 6. Developmental regulatory pathways governing valvulogenesis also control development of cartilage, bone, and tendon 6. Specific shared regulatory pathways include Notch (atrialis/ventricularis & elastic artery development), BMP-Sox9-aggrecan (spongiosa & chondrogenesis), Wnt-periostin (fibrosa & osteogenesis), and FGF-scleraxis-tenascin (chordae tendineae & tendon development). These observations, coupled with pathologic similarities between degenerative valve disease and osteogenesis in the case of CAVD or chondrogenesis in the case of MMVD have led to a working hypothesis that degenerative valve diseases may be recapitulating osteogenesis or chondrogenesis through shared regulatory pathways 6, 7, 8. Recent studies have shown that calcific aortic valves express bone-related markers including Runx2, osteocalcin, osteopontin, and others 9,10. These observations support a hypothesis that calcific aortic valves undergo active osteogenesis, but much less is known about MMVD.
In this study, we tested the hypothesis that myxomatous mitral valves might be undergoing a pathologic process that mimics chondrogenesis. To address this hypothesis we evaluated presence of cartilage-related and bone-related markers including aggrecan (cartilage-abundant PG core), type II collagen (cartilage-specific collagen), Sox9 (chondrogenic transcription factor), and Runx2 (osteogenic transcription factor) in normal and myxomatous human mitral valves.
Materials and Methods
Tissue Collection and Histology:
Surgically-excised myxomatous mitral valves (n = 9) were obtained from human patients with severe primary mitral regurgitation undergoing mitral valve repair or replacement. Anonymous valves were obtained with informed patient consent under an Institutional Review Board approved protocol from the Medical Center of the Rockies (Loveland CO). Normal human mitral valves (n = 3) were obtained from patients without overt cardiovascular disease through the NIH Cooperative Human Tissue Network. The median post-mortem ischemic period collection time for normal mitral valves was 10.5 h (range: 1.5 to 18.2 h), whereas myxomatous valves were obtained immediately after surgery.
The thickness of valve leaflets was measured in 3 locations throughout the samples with calipers. Myxomatous classification was determined by histological analyses by a pathologist and previous methods used by our group 11. Tissues were fixed with 10% formalin, embedded in paraffin, cut into 4 µm sections, and mounted on glass slides. Each valve was stained with hematoxylin-eosin and modified Movat pentachrome stain for histopathologic evaluation. Valves were evaluated for morphologic changes including PG/GAG accumulation, disruption of collagen bundles, elastin fragmentation, and loss of normal valve architecture.
Immunohistochemistry:
Immunohistochemistry was performed to determine the presence of Runx2, Sox9, type II collagen and aggrecan. Immunohistochemistry staining was performed as per previously published methods 12. Briefly, slides underwent deparaffinization, rehydration, PG/GAG chain digestion with chondroitinase ABC (200 mU/mL for 30 min at 37 oC, Seikagaku Co, Tokyo, Japan) and heat-induced epitope retrieval (HIER) with citrate buffer (pH 6) at 95 oC for 20 min (when applicable). Tris-buffered saline (TBS) was used to dilute enzymes, sera and antibodies. Immunohistochemical staining was performed using an automated stainer. Sections were incubated with 10% goat serum followed by 0.03% hydrogen peroxide for 15 min prior to incubation with the primary antibodies (30 - 60 min). Primary anti-mouse monoclonal antibodies and respective titers were: aggrecan 1:100, collagen 2A1 1:25 with HIER, Runx2 1:50, and Sox9 1:50 (all Santa Cruz Biotechnology, Inc, Santa Cruz CA, except for Runx2 from Abcam, Cambridge MA). The anti-mouse peroxidase-labeled polymer (Envision+, Dako, Carpinteria CA) was then applied for 30 min and peroxidase activity was visualized with 3,3-diaminobenzidine. Slides were rinsed in TBS containing 0.01% Tween-20 after each incubation step. Sections were counterstained with Mayer’s haematoxylin (Dako, Carpinteria CA), dehydrated and mounted. Negative controls were created by substituting universal negative control mouse antibodies for primary monoclonal antibodies.
Data Analysis:
Slides were photographed at low-magnification and areas of positive aggrecan staining were quantified using color thresholding in the ImageJ software 13. Statistical differences in frequency of immunopositive staining between normal and myxomatous valves were determined by the Fisher’s exact test. Values of p < 0.05 were considered significant.
Results
Study Population:
The median ages of the normal control group (n = 3) and myxomatous group (n = 9) patients were 45 years (range: 18 to 55 years) and 66 years (range: 34 to 84 years), respectively. Four patients were female (3 normal and 1 myxomatous) and eight were male (0 normal and 8 myxomatous). Normal mitral valves were all from anterior leaflets. Of the myxomatous mitral valves, 4 were anterior leaflets and 5 were posterior leaflets. Cardiovascular comorbidities in the myxomatous group were: atrial fibrillation (n = 2), systemic hypertension (n = 2), and coronary artery disease (n = 2).
Pathology:
The mean thickness of myxomatous mitral valves (2.01 ± 0.76 mm) was increased (p <0.05, Student’s t test) compared to normal mitral valves (0.75 ± 0.09 mm). Normal leaflet stratification was lost in myxomatous mitral valves. The entire leaflet was thickened, but often not uniformly. Expansion of the leaflet was due primarily to regionally extensive areas of basophilic myxomatous matrix. Movat pentachrome stain revealed that the thickening was due to increased glycosaminoglycan (GAG) (Figure 1). Change was most prominent in the laminae spongiosa and fibrosa. Myxomatous valves demonstrated increased elastic fibers that were disorganized and fragmented. Calcification was not present in myxomatous valves.
Figure 1.Low-magnification (scale bars = 1 mm) Movat pentachrome (A and C) and aggrecan immunohistochemistry (B and D) photomicrographs of normal (A and B) and myxomatous (C and D) mitral valves demonstrating focal aggrecan expression and co-localization of aggrecan expression with areas of myxomatous change in myxomatous mitral valves. High-magnification (scale bars = 50 μm) photomicrographs of sequential sections from a myxomatous valve demonstrating co-localization of myxomatous change with cartilage-like cells (E), aggrecan (F), type II collagen (G), and Sox9 (H).
Immunohistochemistry:
Results for immunohistochemical staining of normal and myxomatous mitral valves are summarized in Table 1. All myxomatous mitral valves demonstrated discrete focal areas of staining for aggrecan (Figure 1D and Figure 1F, compared to Figure 1B). The mean area of positive aggrecan staining in myxomatous mitral valves was 14.2% ± 8.3%. Areas of aggrecan immunopositive staining co-localized with areas of myxomatous change on sequential Movat stained sections, however not all areas of myxomatous change were characterized by aggrecan staining. Normal mitral valves were essentially negative for aggrecan staining with a mean staining area of 0.2% ± 0.1%. Aggrecan is the prototypical and most abundant PG core in cartilage 14 and its expression supports a focal chondrogenic process in myxomatous mitral valves.
Table 1. Frequency of pathologic change and immunopositive staining for chondrogenic (aggrecan, type II collagen, Sox9) and osteogenic (Runx2) markers in normal and myxomatous mitral valves.| Marker | Normal | Myxomatous | p-value |
|---|---|---|---|
| Aggrecan | 0/3 | 9/9 | 0.0045* |
| Type II collagen | 0/3 | 7/9 | 0.0426* |
| Sox9 | 1/3 | 9/9 | 0.0455* |
| Runx2 | 1/3 | 3/9 | 0.7636 |
| GAG deposition | 0/3 | 9/9 | 0.0045* |
| ECM disorganization** | 0/3 | 9/9 | 0.0045* |
Seven of nine myxomatous mitral valves were immunopositive for type II collagen. In 5 myxomatous valves, type II collagen strongly co-localized with focal areas of aggrecan staining on sequential histologic sections (Figure 1). Type II collagen staining was not present in normal mitral valves. Type II collagen is the prototype collagen for cartilage and is considered specific for cartilaginous tissues 15. Co-localization of type II collagen and aggrecan constitutes further evidence for a focal pathologic process that mimics chondrogenesis in myxomatous mitral valves.
Sox9 and Runx2 are transcription factors involved in early chondrogenesis and late chondrogenesis / early osteogenesis, respectively 16. All myxomatous mitral valves were immunopositive for the chondrogenic transcription factor Sox9. In all myxomatous mitral valve leaflets, Sox9 co-localized with focal aggrecan-positive areas (Figure 1H). Staining for the osteogenic transcription factor, Runx2, was not prominent in normal or myxomatous mitral valve leaflets. One normal and 3 myxomatous mitral valves exhibited small foci of Runx2 immunopositive cells that did not co-localize with areas of aggrecan staining or myxomatous pathology in myxomatous valves. In the remaining valves, immunopositive staining for Runx2 was limited to a few (< 0.1%) interstitial cells randomly distributed throughout all mitral valves. The later valves were counted as negative for Runx2 for statistical purposes (Figure 2).
Figure 2.Photomicrographs of Runx2 immunohistochemistry of a normal (A) and myxomatous (B) mitral valve demonstrating minimal staining for Runx2. Scale bars = 25 μm.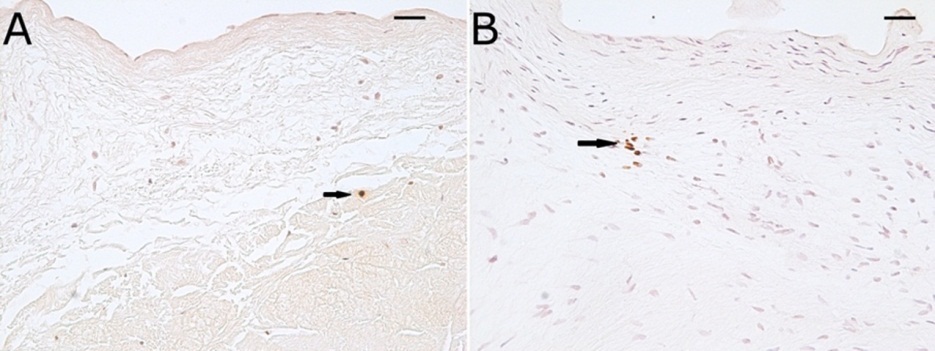
Discussion
The cartilage-related markers studied here interconnect during the earlier phase of chondrogenesis, which chondrocytes are proliferating. These chondrocytes strongly up-regulate the expression and activity of the transcription factor Sox9, inducing important downstream targets such as type II collagen and aggrecan 17. In addition, cells with high levels of Sox9 have the ability of down-regulating Runx2 by directing its degradation, thus preventing or delaying osteogenesis 18.
Proteoglycans consist of a core protein with a variable number of covalently bound GAG side-chains 19. Principal PG cores in normal mitral valves include decorin, biglycan, and versican; and not surprisingly these and other PG cores are more abundant in myxomatous mitral valves 20,21, 22,23. Aggrecan is the prototypic PG core in cartilage 19. Aggrecan has one of the largest PG core proteins and approximately 100 GAG side-chains of varying composition depending on the species 19. This structure makes aggrecan highly hydroscopic and largely gives cartilage its unique biomechanical properties to resist compressive forces. Expression of aggrecan has been previously reported in developing heart valves 8, but is not known to be present in adult mitral valves. This study demonstrated discrete focal expression of aggrecan in myxomatous, but not normal, mitral valves. These focal areas of aggrecan expression correlated strongly with focal areas of myxomatous pathology in myxomatous mitral valves based on Movat staining, but aggrecan was not present in all areas of myxomatous change. The spatial distribution of PG and GAG profiles in mitral valves is not uniform and is likely influenced by different mechanical forces (e.g. tension vs. compression) within the valve apparatus 20,21,22,23,24. Interstitial cells in aggrecan-positive areas often exhibited a rounded hypertrophic morphology reminiscent of chondrocytes that has been reported previously in human myxomatous mitral valves 9.
Collagen is a prominent component of the ECM of heart valves, particularly the lamina fibrosa. The most abundant collagen types in adult heart valves are types I and III 25,26. Despite net degradation and disarray of collagen in MMVD, types I and III collagen are more abundant in myxomatous mitral valves 25. Type II collagen is the most abundant collagen in cartilage and its expression is considered largely specific for cartilaginous tissues 27. Type II collagen is expressed in the heart during the development of endocardial cushions, but not in the adult heart 6. In this study, focal presence of type II collagen in aggrecan-abundant areas of myxomatous change constitutes further compelling evidence for a chondrogenic process in myxomatous mitral valves. Type II collagen has been previously identified in adult calcific aortic valves, largely associated with areas of osteogenic transformation 10. This finding supports the notion that the osteogenic process in CAVD recapitulates endochondral bone formation wherein bone formation progresses through a process of ossification of a cartilaginous template 9.
The transcription factor Sox9 is typically expressed in chondroprogenitors, periarticular chondrocytes and proliferating chondrocytes. Runx2, which serves as a positive regulatory factor in chondrocyte maturation, is expressed in hypertrophic chondrocytes and throughout ossification stages. Thus, their regulatory mechanisms follow a temporal pattern during endochondral ossification. 28,29. A variety of signaling pathways promote their regulation 30 with chondrogenesis being favored by high Sox9 and low Runx2 expression and osteogenesis being favored by low Sox9 and high Runx2 expression 29. In this study, we observed strong focal presence of Sox9 in myxomatous mitral valves that co-localized with focal areas of aggrecan and type II collagen positive staining. This suggests that focal chondrogenesis in myxomatous mitral valves may be mediated in part through the Sox9 transcription factor. Bone morphogenetic protein (BMP) and transforming growth factor beta (TFGβ) are members of the same signaling superfamily. Both are known to regulate Sox9 in late-stage chondrogenesis 31, 32, 33. Increased abundance of TGFβ has been demonstrated in canine myxomatous mitral valves suggesting a role for TGFβ in a chondrogenic-like pathology in degenerative mitral valve , possibly through regulation of Sox9 34, 35,36. BMP2 induces Sox9 and aggrecan expression in developing heart valve progenitor cells 37. BMP-Sox9 is postulated to mediate valvular heart diseases through recapitulation of shared developmental pathways that regulate valvulogenesis and chondrogenesis but this hypothesis has not been yet confirmed 6,7,8. Lastly, studies have demonstrated direct cross-talk between BMP and Wnt canonical signaling during chondrogenesis 38,39. Possible roles for BMP or Wnt signaling in degenerative valvular heart disease remain to be elucidated.
Strong Runx2 expression has been demonstrated in calcific aortic valves and has thus been implicated in an osteogenic pathologic process in CAVD 9,10. Runx2 was not strongly expressed in either normal or myxomatous mitral valves suggesting that it does not play a significant role in MMVD. Sox9 expression has also been demonstrated in calcific aortic valves 9,10. Cells that express Sox9 can be progenitors for a variety of cell types, including chondrocytes and osteoblasts 40. During skeletogenesis, bone formation occurs through endochondral ossification of a cartilage template. Endochondral ossification proceeds through an early phase of Sox9 expression during chondrocyte proliferation and a late phase of Runx2 expression during chondrocyte hypertrophy and bone formation 28. Thus, it can be suggested that CAVD disease mimics an endochondral osteogenesis process, whereas MMVD disease recapitulates chondrogenesis only.
Limitations of this study are the low relatively low number of normal mitral valves available for study and the fact that they were not perfectly age-matched to the myxomatous mitral valve group. Despite the low sample number of the normal control, statistical significance was reached in frequency positive straining for chondrogenic markers because of their virtual absence from normal mitral valves. The lack of perfect age-matching between the normal control group and the myxomatous group raises the possibility that age could influence expression of chondrogenic makers. The absence of availability of posterior leaflets in the normal group is also a limitation. However, we did not observe differences between anterior and posterior leaflets in the myxomatous group.
Conclusion
In conclusion, focal co-expression of aggrecan, type II collagen, and Sox9, but not Runx2, suggest a pathologic process that mimics chondrogenesis in human myxomatous mitral valves. The signaling pathways that mediate this process and the reasons why MMVD and CAVD apparently differ in their pathology remain to be determined.
Acknowledgements
The authors gratefully acknowledge the Human Cooperative Tissue Network and Ms. Kathy Kioussopoulos, Dr. Mark Guadagnoli and Dr. Fernando Lamounier of the Medical Center of the Rockies for their assistance in obtaining human heart valves.
Abbreviations
bone morphogenetic protein
CAVDcalcific aortic valve disease
ECMextracellular matrix
GAGglycosaminoglycan
HIERheat-induced epitope retrieval
MMVDmyxomatous mitral valve disease
PGproteoglycan
TBStris-buffered saline
TGFβtransforming growth factor beta.
References
- 1.Iung B, Vahanian A.Epidemiology of valvular heart disease in the adult. , Nat Rev Cardiol 2011, 162-72.
- 2.Nkomo V T, Gardin J M, Skelton T N, Gottdiener J S, Scott C G et al.Burden of valvular heart diseases: a population-based study. , Lancet 2006, 1005-11.
- 3.Schoen F J.Cardiac valves and valvular pathology: update on function, disease, repair, and replacement. Cardiovasc Pathol. 2005, 189-94.
- 4.Pellerin D, Brecker S, Veyrat C.Degenerative mitral valve disease with emphasis on mitral valve prolapse. Heart. 2002;88 Suppl 4-20.
- 5.Otto C M.Valvular aortic stenosis - Disease severity and timing of intervention. , Journal of the American College of Cardiology 2006, 2141-51.
- 6.Combs M D, Yutzey K E.Heart valve development: regulatory networks in development and disease. Circ Res. 2009, 408-21.
- 7.Markwald R R, Norris R A, Moreno-Rodriguez R, Levine R A.Developmental basis of adult cardiovascular diseases: valvular heart diseases. , Ann N Y Acad Sci 2010, 177-83.
- 8.Lincoln J, Lange A W, Yutzey K E.Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. , Dev Biol 2006, 292-302.
- 9.Caira F C, Stock S R, Gleason T G, McGee E C, Huang J et al.Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. , J Am Coll Cardiol 2006, 1707-12.
- 10.Wirrig E E, Hinton R B, Yutzey K E.Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. , J Mol Cell Cardiol 2011, 561-9.
- 11.Disatian S, Lacerda C, Orton E C.Tryptophan hydroxylase 1 expression is increased in phenotype-altered canine and human degenerative myxomatous mitral valves. The Journal of heart valve disease. 2010, 71-8.
- 12.Disatian S, Ehrhart E J, Zimmerman S 3rd, Orton E C.Interstitial cells from dogs with naturally occurring myxomatous mitral valve disease undergo phenotype transformation. , J Heart Valve Dis 2008, 402-11.
- 14.Bayliss M T, Howat S, Davidson C, Dudhia J.The organization of aggrecan in human articular cartilage. Evidence for age-related changes in the rate of aggregation of newly synthesized molecules. The Journal of biological chemistry. 2000, 6321-7.
- 16.Seibel M J, Robins S P, Bilezikian J P. (2006) Dynamics of bone and cartilage metabolism. 2nd ed. San Diego:.
- 17.Dong Y, Jesse A M, Kohn A, Gunnell L M, Honjo T et al.RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. , Development 2010, 1461-71.
- 18.Cheng A.Genever PG. SOX9 determines RUNX2 transactivity by directing intracellular degradation. , J Bone Miner Res 2010, 2680-9.
- 19.Esko J, Kimata k, Lindahl U. (1999) Proteogycans and sulfated glycosaminoglycans. Essentials of Glycobiology In: Valki A, Cummings R, Esko J, Freeze H, Hart G, Marth J, editors .
- 20.Grande-Allen K J, Calabro A, Gupta V, Wight T N, Hascall V C et al.Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. , Glycobiology 2004, 621-33.
- 21.Stephens E H, Chu C K, Grande-Allen K J.Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. , Acta Biomater 2008, 1148-60.
- 22.Gupta V, Barzilla J E, Mendez J S, Stephens E H, Lee E L et al. (2009) Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol.
- 23.Lacerda C M, Disatian S, Orton E C.Differential protein expression between normal, early-stage, and late-stage myxomatous mitral valves from dogs. Proteomics Clin Appl. 2009, 1422-9.
- 24.Grande-Allen K J, Griffin B P, Ratliff N B, Cosgrove D M, Vesely I.Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. , J Am Coll Cardiol 2003, 271-7.
- 25.Cole W G, Chan D, Hickey A J, Wilcken D E.Collagen composition of normal and myxomatous human mitral heart valves. , Biochem J 1984, 451-60.
- 26.Latif N, Sarathchandra P, Taylor P M, Antoniw J, Yacoub M H.Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. 2005, 218-27.
- 27.Mwale F, Stachura D, Roughley P, Antoniou J.Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. , J Orthop Res 2006, 1791-8.
- 28.Goldring M B, Tsuchimochi K, Ijiri K.The control of chondrogenesis. , Journal of cellular biochemistry 2006, 33-44.
- 29.Kelly D J, Jacobs C R.The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth defects research Part C, Embryo today : reviews. 2010, 75-85.
- 30.Itasaki N, Hoppler S.Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Developmental dynamics : an official publication of the American Association of Anatomists. 2010, 16-33.
- 31.Montero J A, Lorda-Diez C I, Ganan Y, Macias D, Hurle J M.Activin/TGFbeta and BMP crosstalk determines digit chondrogenesis. , Dev Biol 2008, 343-56.
- 32.Keller B, Yang T, Chen Y, Munivez E, Bertin T et al. (2011) Interaction of TGFbeta and BMP signaling pathways during chondrogenesis. PLoS One.
- 33.Karamboulas K, Dranse H J, Underhill T M.Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFbeta signals. , J Cell Sci 2010, 2068-76.
- 34.Obayashi K, Miyagawa-Tomita S, Matsumoto H, Koyama H, Nakanishi T et al.Effects of transforming growth factor-beta3 and matrix metalloproteinase-3 on the pathogenesis of chronic mitral valvular disease in dogs. , Am J Vet Res 2011, 194-202.
- 35.Aupperle H, Marz I, Thielebein J, Schoon H A.Expression of transforming growth factor-beta1, -beta2 and -beta3 in normal and diseased canine mitral valves. , J Comp Pathol 2008, 97-107.
- 36.Disatian S, Orton E C.Autocrine serotonin and transforming growth factor beta 1 signaling mediates spontaneous myxomatous mitral valve disease. , J Heart Valve Dis 2009, 44-51.
- 37.Lincoln J, Alfieri C M, Yutzey K E.BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. , Dev Biol 2006, 292-302.
- 38.Tuan R S.Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. , J Bone Joint Surg Am. 2003;85-A Suppl 2, 137-41.
Cited by (3)
- 1.Wang Xinmei, Kuban-Johnston Danielle, Lapuerta Pablo, Lacerda Carla M. R., 2022, Telotristat ethyl reverses myxomatous changes in mice mitral valves, Frontiers in Cardiovascular Medicine, 9(), 10.3389/fcvm.2022.945672
- 2.Fang Ming, Alfieri Christina M., Hulin Alexia, Conway Simon J., Yutzey Katherine E., 2014, Loss of β-Catenin Promotes Chondrogenic Differentiation of Aortic Valve Interstitial Cells, Arteriosclerosis, Thrombosis, and Vascular Biology, 34(12), 2601, 10.1161/ATVBAHA.114.304579
- 3.Koch Christopher D., Lee Chan Mi, Apte Suneel S., 2020, Aggrecan in Cardiovascular Development and Disease, Journal of Histochemistry & Cytochemistry, 68(11), 777, 10.1369/0022155420952902
