Abstract
Over the last few decades, many research works highlighted the role of miRNAs on cardiac diseases. Ischaemic heart disease (IHD) or coronary heart disease is a condition that is mainly caused by atherosclerosis. It has been established that microribonucleic acids regulate many factors that are involved in the development and pathophysiology of IHD. As a result, there are great potential opportunities for miRNAs to be used as a biomarker for disease differentiation, as well as novel drug targets or therapeutics for the treatment and also as a diagnostic approach. As it is now evident that miRNAs play critical roles in the disease mechanisms, this review article tried to focus on the pathway, in which; the miRNAs stimulate the IHD to develop. By understanding the mechanisms, it will be possible to present a complete strategy of IHD treatment and also solving all the impediments that are highlighted in this article. Still, there are a number of limitations and obstacles on the way of developing a proper therapeutic approach that can be approved and well accepted. This review is mainly dependent on the potential of miRNAs as a promising arena on the field of cardiac treatment and the possible obstacles that are needed to be explored and overcome.
Author Contributions
Academic Editor: HatoriNobuo, Director, Kobayashi Hospital
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Sarmistha Mitra, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The existence of miRNAs is a relatively recent discovery; however, recent works suggested that miRNA can be related with the pathophysiological development of various cardiovascular diseases 1 like ischaemic heart disease 2. The initial discovery of miRNA was in 1993 3 and now, it is proved that miRNAs are endogenous ~22-nucleotide (nt) noncoding RNAs 4, have the ability to control the post-transcriptional regulation of gene expression via translational inhibition or degradation of target mRNAs 5, and therefore have a key role in the disease development process 6. Dysregulation of mRNA depend on the degree of complementarity between the 5′ seed sequence (5–7 nt) of the miRNA and the 3′ untranslated region (UTR) of the target mRNA 7. Thus, miRNAs are associated with the mechanism of differentiation, proliferation, electrical conduction, angiogenesis and apoptosis 8. These pathways are the major reasons behind the different physiological and pathological adaptations of various diseases 9. Till to date, about 2000 miRNAs have been discovered, and they are known to regulate the expressions of one third of human genes 10. These ever-growing researches on mi-RNA discovery therefore suggested miRNA as a therapeutic target, as the regulation of cell development, proliferation, differentiation, apoptosis and metabolism can be modulated by altering mi-RNA level 11, 12.
In the present time, cardiovascular disease is one of the most major reasons of mortality in the developed country, causing 16.7 million deaths in each year 13, 14. In this estimation, only Ischemic Heart Disease (IHD) alone contributes to over 7 000 000 deaths in the USA 15. The function of miRNAs in IHD is being substantially investigated rigorously to find out a new way for diagnosis of pathophysiology of the damaged tissues. So, the evaluation of miRNAs will provide not only new insights into the pathophysiology of tissue injury but also the potential of cardiac biomarkers 16, 17 to diagnose 18 diseases and offersa real-time glimpse of the progression 19 of the disease. Therefore, in this article, we aimed to emphasize the involvement of different miRNAs in IHD, describing their significant therapeutic benefits in the field of diagnosis and treatment and also discussed the prospects of miRNAs in the near future.
Role of mi-RNAs in Cardiac Development
In the embryonic stage of the animal, heart is the primary organs that firstly developed and function for fatal life. The development process initiates with the construction of two endocardial tubes, which are fused afterwards to form primitive heart tube that septates into four chambers and paired arterial trunks to form adult heart 20. During this process, different mi-RNAs play functional roles in different stages of cardiac formation 21, 22. Figure 1 summarized the involvement miRNAs in different stages of cardiac development. As an example, miR-1 target different genes in the heart 23, where its over expression leads to the inhibition of cardiac hypertrophy 24 and thereby confirms the ventricular myocyte proliferation 25. While deletion of its family member miR-1-2 caused a wide range of cardiac abnormalities 26. Combination of heart field is promoted by miR-218, and it is also involved in transformation of cardiac crescent to linear heart tube. Furthermore, heart looping is initiated by miR-138 21, and also involved in the formation of atrioventricular canal from linear heart tube. In collaborative approach, miR-1, miR-133 and miR-17-92 perform embryonic heart maturation and septation, where post-natal heart growth and maturation is accomplished by miR-15 27. Under stress condition, the involvement of miR-208a has been also reported to be required in cardiac growth 16. Beside above, miR-15, miR-133, miR-199, miR-590 are involved in cardiomyocyte proliferation, in which miR-133, miR-1, and miR-499 caused progenitor and stem cell differentiation and thus involved direct reprogramming of fibro blast to myocytes 27, 28. In cardiogenesis, pattern of chambers and valve region is maintained by miR-138, while miR-21 is appeared to necessary in atrioventricular valve development and it expressed in valvular endothelium. MiR-208a involves in apt development of the cardiac conduction system 28, 29. Other miRNAs, such as miR-23, miR-126, miR-143, miR-199, and miR-208b are also engaged to participate in cardiac development, functions, or diseases 30, 31. As this miRNAs, can be called ‘cardiac miRNAs’, are involved in the various development processes of the heart formation, dysregulations of these miRNAs may cause the IHD, which will be discussed in further section.
Figure 1.Illustration of miRNAs role in the three stages of cardiac development. Here, deep blue color represents the cluster of miRNAs, and green deep blue color denotes the miRNA family.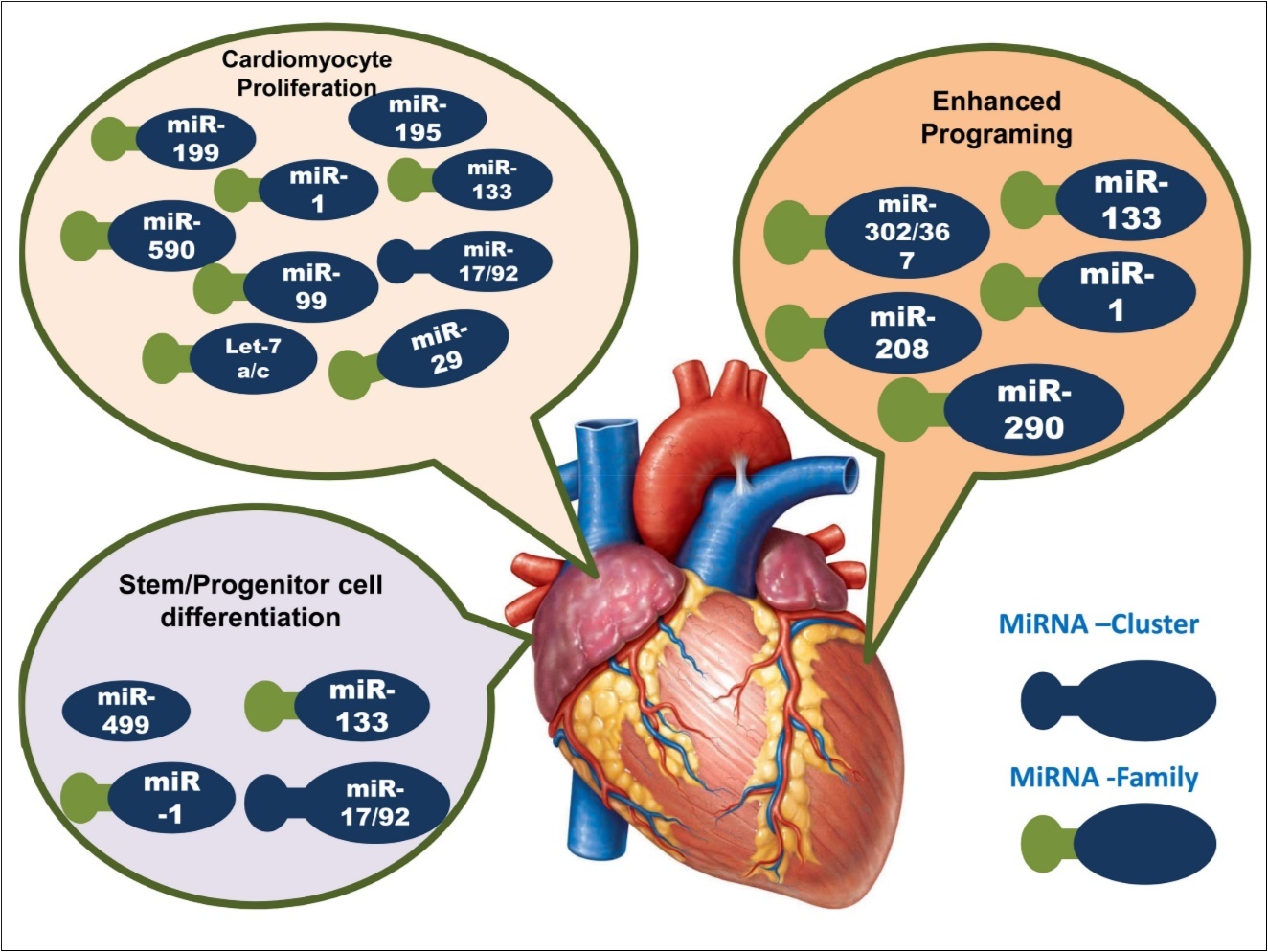
miRNAs in Ischemic Heart Disease (IHD)
The root of ischemic disease can be considered as atherosclerosis, a process in which, lipids and fibrous elements are accumulated in the arteries, and progressively atherosclerotic plaque formed. Plaque formation is developed by lipids and leukocyte infiltration, where the degree of influx is determined based on monocyte recruitment, macrophage egress to atherosclerotic lesions and also the balance of proliferation, survival, and apoptosis within the arterial walls 32. In the first step, endothelial dysfunction is occurred, and allow leukocyte and monocyte infiltration into the vessel wall. The major steps involved in ACS are foam cell formation, plaque angiogenesis, migration and proliferation of vascular smooth muscle cells (VSMC), fibrous cap destabilization and plaque rupture, and thrombus formation on destabilized plaque which finally leads to acute coronary syndromes (ACS). It is evident that miRNAs are involved in all steps 33, 34.
As shown in Figure 2, in endothelial cell activation step, miR-21 is involved which inhibits the expression of PTEN 35, 36 and PPARα 37, 38 (Peroxisome proliferator-activated receptor alpha). A number of miRNAs are involved in the next step including miR-155 6, 39, miR-124 6, 40, miR-146 6, 41, 42, miR-147 43, miR-223 44, miR-106a 45, miR-20a 46, miR-125a 6, 37. During cholesterol biosynthesis, miR-122 and miR-33 are also involved. miR-33 regulates expressions of ABCA1 47, ABCG1 48, CPT1α 37, 48. In plaque angiogenesis step, miR-126 49, 50, miR-92a 51, miR-221 52, miR-222 52, miR-23 53, miR-24 54, 55, miR-27, miR-1 are reported to be implicated 56. In the final stage of atherosclerosis, miR-143/145 57, 58, miR-221/222 52, 53, miR-195 59, miR-100 60, miR-132 61, miR-133 are reported to regulating some targets that can be beneficial (indicated by green color) or detrimental (red color) for disease condition.
Figure 2.Involvements of miRNAs in different stages of atherosclerosis. Here, atheroprotective miRNAs are shown in green color and red color represents the atherogenic miRNAs. The targets that are stimulated by miRNAs are indicated by deep blue color box while, the targets inhibited by miRNAs are denoted as light blue color.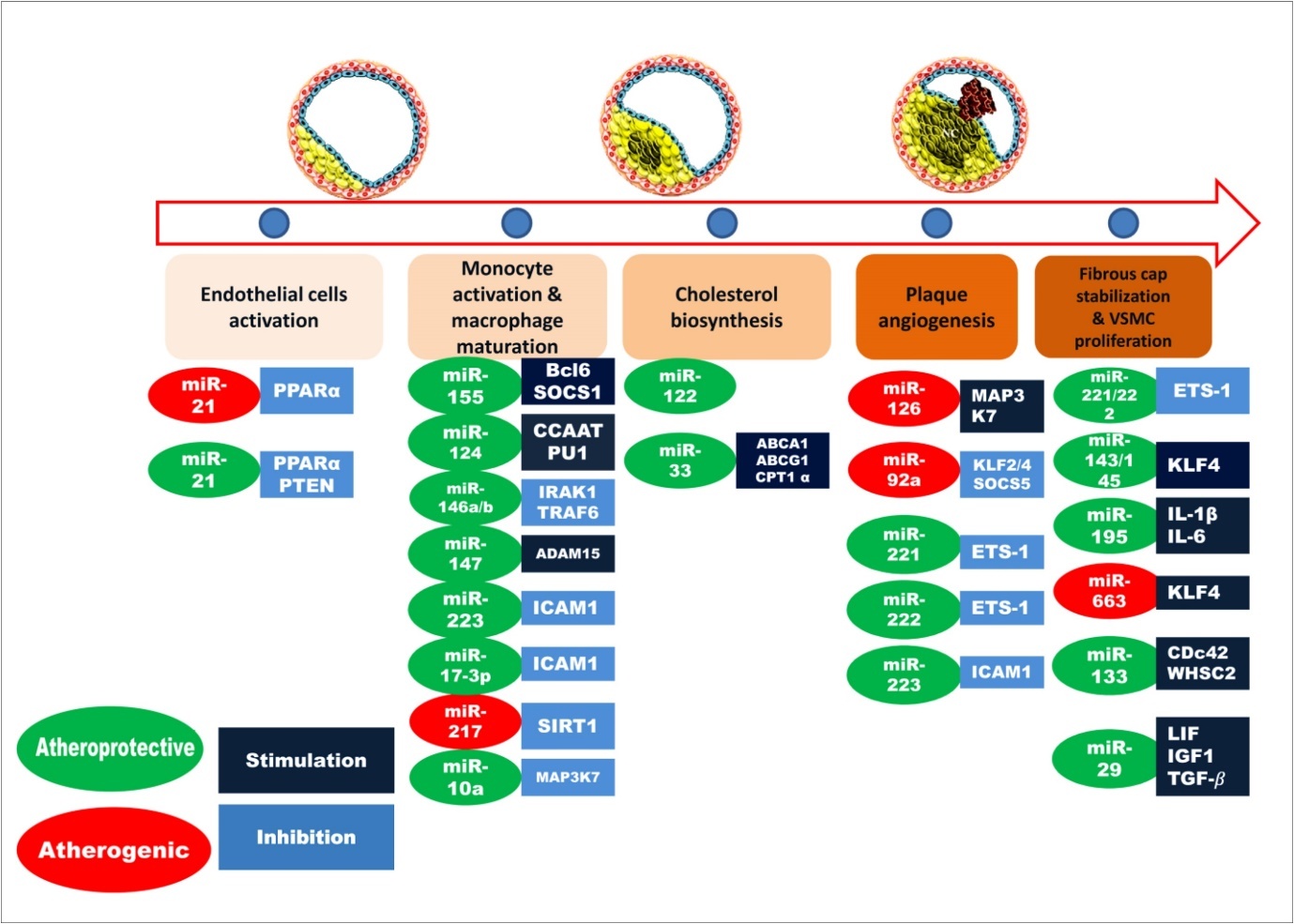
One miRNA can be highly expressed in one tissue, but may have no or low expression in other tissues 62, 63. miR-29 is involved in fibrotic reaction after myocardial infarction 64, 65, while miR-21 66, 67 may exert a fundamental role in post-angioplasty restenosis. Overexpression of miR-21, following I/R injury, decreased injured area and reduced cell apoptosis in border area, which improved myocardial collagen Ⅰ, Ⅲ remodeling in noninfarcted area, as well as improved heart function and hemodynamic status, and inhibited left ventricular remodeling 68. miR-208 is also involved in the shifting toward gene expression pattern in contractile proteins in heart failure. Moreover, miR-1 influences susceptibility to cardiac arrhythmias after myocardial infarction 64. It is also observed that only miR-320 expression was consistently decreased in murine hearts on ischaemic or reperfusion injury in vivo and ex vivo. So it can be said that overexpression of miR-320 can cause increased sensitivity to I/R injury 9 and therefore, a potential target for IHD therapy, as it is responsible for negative regulation of Hsp20 translation. Overexpressions of miR-23a, miR-23b, miR-24, miR-195 or miR-214 via adenovirus-mediated gene transfer increased hypertrophic growth of cultured cardiomyocytes and may cause heart failure 69, 70, whereas overexpression of miR-150 or miR-181b caused a decrease in cardiomyocyte cell size 16, 63, 70. In many recent studies, it is observed that circulating miRNAs can be responsible for heart failure. miRNAs with altered circulating levels in patients caused heart failure are including miR-122, miR-210, miR-423-5p, miR-499 and miR-62 71, 72, 73. There are also some other miRNA listed, which may have some beneficial effects after the injury. miRNA-101 regulates multiple proteins, including collagens and fibrillins. During fibrosis these miRNA could represents future therapeutic targets to activate the fibrotic response after heart injury 74, 75. miR-34 is a critical modulator of cardiac biological pathways, including cardiomyocyte death, senescence and proliferation 76, 77.
Recently it has been analyzed that combining multiple miRNAs into a miRNA profile can provide greater accuracy than can be expected from the assessment of a single miRNA. Prospective large-scale studies are needed to define the true potential of circulating miRNAs as biomarkers for atherosclerotic-related disease states and diagnosis of these diseases. For example, it has been established that miR-1 is a potential biomarker for early diagnosis of acute Myocardial infarction to differentiate it from other cardiac diseases 78. Deletion of the miR-1 may cause serious cardiac defects. Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor function are the main targets of miR-1 37, 79.
In recent work it is found that miR-146a is another cytokine-responsive miRNAs that provides significant atheroprotective properties in the vessel wall. Cytokines such as TNF-α and IL-1β induced expression of miR-146a and miR-146b in a delayed kinetic manner in endothelial cells (ECs) that coincided with the resolution of inflammatory gene expression 80. The overexpression of miR-146a inhibited cytokine responsiveness in ECs, which suggests that it may participate in a negative feedback mechanism to reduce EC inflammatory signaling. Indeed, miR-146a repressed both NF-κB and MAPK signaling pathways by directly targeting HuR, an RNA-binding protein that elicits inhibitory effects on endothelial nitric oxide synthase (eNOS). In addition, miR-146a represses the induction of EC adhesion molecules by targeting upstream adaptor proteins TRAF6 and IRAK1/2.In contrast to the more selective inhibitory role of miR-181b on EC NF-κB signaling, miR-146a inhibits NF-κB signaling in both ECs and macrophage s81. So miR-146 is enlisted as an important cytokine-responsive miRNA that may serves to reduce EC inflammation.
Future Prospects of miRNA Based Therapy
The involvement of miRNAs in controlling regulation of various targets of cardiovascular diseases has emerged a potential research field. It is well established from above studies that several miRNAs have very significant regulatory impact on atherosclerotic processes in biological system, but it is not absolutely known how broad the contribution of individual miRNAs is. Furthermore, in most of the animal studies to date, the phenotypic effects of miRNA inhibition have been observed, explored and analyzed only in the target tissue of interest, but the effects on other tissues, targets and pathways are not yet have been explored. As a small number of miRNAs are proved to be target-specific, so delivering of miRNA mimics or inhibitors in a targeted manner may provide a potential effect on the treatment of atherosclerosis rather than delivering in a systemic manner. Again it has an added advantage which enables to act on more than one target at the same time and may give more rapid effects than targeting only one. As the scopes to examine and explore miRNA’s function in complex biologic systems are increasing day by day with the advancement of tools and other animal models; in the near future, it will be possible to have a broader and more cleared picture on the role of these regulatory molecules in specific stages of atherosclerotic lesion formation and their absolute and specific function on cardiac disease treatment. As miRNA targets multiple genes involved in same pathway in disease progression, it can control the cellular function by inhibiting and degrading mRNAs or their translation. However, thesystemic delivery of miRNA as therapeutic may rise as a big challenge; because of the possibility of acting on a number of targets which increases the risk of unwanted side effects.
The involvement of miRNAs in cardiac development therefore, opens a new era for IHD treatment, as they are linked to the development and progression of cardiovascular diseases. As an example, miRNAs found in system circulation can be considered as promising new biomarker, since they have many properties that can easily detectable and accessible from extracellular fluids by quantitative reverse-transcription polymeric chain reaction or microarray etc, also their change patterns are disease specific. Eventually, decreased plasma levels of miR-17, miR-92a, miR-126, miR-181b, miR-145, miR-155 82 are associated with coronary artery disease 37. These biomarkers can be very effective for diagnosis and differentiation of various cardiac problems. So the future research works must be designed to find out the expression pattern and nature of miRNA on various cardiac diseases.
Pharmacological targeting of dysregulated miRNAs is a rising and very significant concept that has important therapeutic potential. The multiplicity of miRNA targets enables miRNAs to bypass mechanisms that render cells or tissues insensitive to certain drugs. For example, it is possible for the cells to develop insensitivity to single drugs by initiating rare mutations in drug targets or desensitizing the receptors of cell surface. miRNA, which targets a number of steps in a disease pathway are possibly not affected by this kind of mechanisms and are very efficient to be applied in field of development of new therapeutic strategy. An efficient and potential therapeutic strategy may be developed by the delivery of a cassette of miRNA mimics or inhibitors to work on specific steps of atherosclerotic progression. Therapies designer on the basis of miRNA inhibitors, like locked nucleic acids, antagomiRs, and miRNA sponges, or miRNA mimics are now being developed to repress pathological miRNAs or overexpress protective miRNAs, respectively.
Although most of the miRNA therapeutics is still in preclinical development, only two of them have reached clinical trials. The first miRNA-targeted therapy was a locked nucleotide acid-based antisense miR-122 inhibitor (miRavirsen). Another is one liposome-based miR-34 mimic (MRX34), which has entered a clinical Phase I trial in patients with liver cancer 83, 84. miRNA replacement therapies and anti-miRNA oligonucleotides may be taken up more efficiently in the liver and kidney, but many additional peripheral tissues, such as vessels and heart have also been successfully targeted using currently available delivery approaches. However, much additional works are needed to prove, whether this therapeutic manipulation of miRNA function may indeed represent a safe atherosclerosis treatment in human’s biological system.
It is possible to manipulate miRNAs and obtain therapeutic effect, since they regulate specific targets in a particular cellular pathway, at the same time, it is simple to synthesize short oligonucleotides by interfering mechanism of action of miRNA 64. Over the course of IR injury, there is diminished cardiac function secondary to necrotic tissue formation 85, 86. This prompts the secretion of various factors including: endothelin-1 (ET-1), angiotensin II (Ang II), epinephrine (Epi) and miRNAs 86. From the above study, it can be said that these miRNAs can exhibit either protective or detrimental effects via post-transcriptional regulation of mRNAs. The downstream effects of miRNAs depend heavily on their temporal expression during the development of the pathology, as well as the tissue it originated from 86, 87. Clearly, miRNAs have been identified as key regulators of complex biological processes linked to multiple cardiovascular pathologies, including hypertension, atherosclerosis 88, ischaemic heart disease 71. As the miRNAs have the ability to regulate gene expression via RNA-induced silencing complexes, it is possible to find out a new strategy of treatment by targeting them to mRNAs, where they inhibit translation or direct destructive cleavage. And by analyzing the expression patterns of these miRNAs, it may be possible to establish a new target for cardiac research and providing new therapeutics for cardiac disease patients.
Conclusion
It has been proved from recent studies that there are a lot of possibilities to apply miRNA as therapeutics on the field of IHD. It requires more detailed and cleared concept and understanding of the mechanisms of these miRNAs involved in the regulation of disease development, and their effects on initiation of various stages of atherosclerosis. Recent works concentrated on the potential of miRNAs being used as safe therapeutics in cardiac treatment. However, limitations of the fields including, risk of side effects, its effect on other physiological processes, effects on normal cardiac functioning must be studied and explored more by the development of appropriate animal model. In order to developing a new therapeutic as well as diagnostic approach using miRNAs, these demerits must be understood and analyzed; which can make this be more beneficial, efficient and advanced then existing approaches.
References
- 1.Zampetaki A, Willeit P, Drozdov I, Kiechl S, Mayr M. (2011) Profiling of circulating microRNAs: from single biomarkers to re-wired networks.” Cardiovascular research:cvr266.
- 2.Thum T. (2012) MicroRNA therapeutics in cardiovascular medicine.”. , EMBO molecular medicine 4(1), 3-14.
- 3.Alexiou P, Maragkakis M, G L Papadopoulos, Reczko M, A G Hatzigeorgiou. (2009) Lost in translation: an assessment and perspective for computational microRNA target identification.”. , Bioinformatics 25(23), 3049-3055.
- 4.Guo H, N T Ingolia, J S Weissman, D P Bartel. (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels.”. , Nature 466(7308), 835-840.
- 5.Filipowicz W, S N Bhattacharyya, Sonenberg N. (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight?”. , Nature Reviews Genetics 9(2), 102-114.
- 6.Caroli A, M T Cardillo, Galea R, L M Biasucci. (2013) Potential therapeutic role of microRNAs in ischemic heart disease.”. , Journal of cardiology 61(5), 315-32.
- 7.Felekkis K, Touvana E, Stefanou C, Deltas C. (2010) microRNAs: a newly described class of encoded molecules that play a role in health and disease.”. , Hippokratia 14(4), 236-240.
- 8.Abdellatif M. (2012) Differential expression of microRNAs in different disease states.”. , Circulation research 110(4), 638-650.
- 9.Ren X-P, Wu J, Wang X, M A Sartor, Qian J et al. (2009) MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 2. , Circulation 119(17), 2357-2366.
- 10.R C Friedman, Farh KKH, C B Burge, D P Bartel. (2009) Most mammalian mRNAs are conserved targets of microRNAs.”. , Genome research 19(1), 92-105.
- 11.T M Rana. (2007) Illuminating the silence: understanding the structure and function of small RNAs.”. , Nature reviews Molecular cell biology 8(1), 23-36.
- 12.Vasudevan S, Tong Y, J A Steitz. (2007) Switching from repression to activation: microRNAs can up-regulate translation.”. , Science 318(5858), 1931-1934.
- 13.Dahlöf B. (2010) Cardiovascular disease risk factors: epidemiology and risk assessment.” The American journal of cardiology 105(1):. 3-9.
- 14.Singh S, A K Dash. (2008) Radioprotectants: Basic concepts, current status and future directions.”. , Drugs of the Future 33(8), 681.
- 15.Null G By Gary, Carolyn Dean MD, Martin Feldman ND, Rasio Debora, Smith Dorothy.. PhD Download the PDF of this entire report here.”
- 16.E van Rooij, L B Sutherland, Qi X, J A Richardson, Hill J et al. (2007) Control of stress-dependent cardiac growth and gene expression by a microRNA.”. , Science 316(5824), 575-579.
- 17.S K Gupta, Bang C, Thum T. (2010) Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease.” Circulation:. , Cardiovascular Genetics 3(5), 484-488.
- 18.M A Cortez, G A Calin. (2009) MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases.” Expert opinion on biological therapy 9(6):. 703-711.
- 19.B A Dickinson, H M Semus, R L Montgomery, Stack C, P A Latimer et al. (2013) Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension‐induced heart failure.” European journal of heart failure. 15(6), 650-659.
- 20.Moorman A, Webb S, N A Brown, Lamers W, R H Anderson. (2003) Development of the heart:(1) formation of the cardiac chambers and arterial trunks.”. , Heart 89(7), 806-814.
- 21.S U Morton, P J Scherz, K R Cordes, K N Ivey, D Y Stainier et al. (2008) microRNA-138 modulates cardiac patterning during embryonic development.”. Proceedings of the National Academy of Sciences 105(46), 17830-17835.
- 22.Wystub K, Besser J, Bachmann A, Boettger T, Braun T. (2013) miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development.”. , PLoS Genet 9(9), 1003793.
- 23.W D Townley-Tilson, T E Callis, Wang D. (2010) MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease.”. The international journal of biochemistry & cell biology 42(8), 1252-1255.
- 24.Care A, Catalucci D, Felicetti F, Bonci D, Addario A et al. (2007) . MicroRNA-133 controls cardiac hypertrophy.” Nature medicine 13(5), 613-618.
- 25.Zhao Y, Samal E, Srivastava D. (2005) Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis.”. , Nature 436(7048), 214-220.
- 26.K R Cordes, Srivastava D. (2009) MicroRNA regulation of cardiovascular development.”. , Circulation research 104(6), 724-732.
- 27.A H Williams, Valdez G, Moresi V, Qi X, McAnally J et al. (2009) MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice.”. , Science 326(5959), 1549-1554.
- 28.P M Ridker, Cushman M, M J Stampfer, R P Tracy, C H Hennekens. (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men.” New England journal of medicine. 336(14), 973-979.
- 29.Ferdinandy P, Szilvassy Z, G F Baxter. (1998) Adaptation to myocardial stress in disease states: is preconditioning a healthy heart phenomenon?” Trends in pharmacological sciences. 19(6), 223-229.
- 30.Liu N, E N Olson. (2010) MicroRNA regulatory networks in cardiovascular development.”. , Developmental cell 18(4), 510-525.
- 31.Han M, Toli J, Abdellatif M. (2011) MicroRNAs in the cardiovascular system.” Current opinion in cardiology 26(3):. 181-189.
- 32.Gui T, Shimokado A, Sun Y, Akasaka T, Muragaki Y. (2012) Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery.” Mediators of inflammation.
- 33.V G Haver, R H Slart, C J Zeebregts, M P Peppelenbosch, A R.Tio (2010). “Rupture of vulnerable atherosclerotic plaques: microRNAs conducting the orchestra?” Trends in cardiovascular medicine. 20(2), 65-71.
- 34.Martin K, J F O’sullivan, M N.Caplice (2011). “New therapeutic potential of microRNA treatment to target vulnerable atherosclerotic lesions and plaque rupture.” Current opinion in cardiology 26(6):. 569-575.
- 35.Nishiguchi T, Imanishi T, Akasaka T. (2015) MicroRNAs and cardiovascular diseases.” BioMed research international.
- 36.Yang F, Liu W, Yan X, Zhou H, Zhang H et al. (2016) Effects of mir-21 on Cardiac Microvascular Endothelial Cells After Acute Myocardial Infarction in Rats: Role of Phosphatase and Tensin Homolog (PTEN)/Vascular Endothelial Growth Factor (VEGF) Signal Pathway.” Medical science monitor: international medical journal of experimental and clinical research 22:. 3562.
- 37.Andreou I, Sun X, P H Stone, Edelman E R, M W Feinberg. (2015) miRNAs in atherosclerotic plaque initiation, progression, and rupture.” Trends in molecular medicine. 21(5), 307-318.
- 38.Zhou J, Wang K-C, Wu W, Subramaniam S, Shyy JYJ et al. (2011) MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation.”. Proceedings of the National Academy of Sciences 108(25), 10355-10360.
- 39.Caballero-Garrido E, J C Pena-Philippides, Lordkipanidze T, Bragin D, Yang Y et al. (2015) In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke.”. , Journal of Neuroscience 35(36), 12446-12464.
- 40.Ji Q, Ji Y, Peng J, Zhou X, Chen X et al. (2016) . Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients.” PloS one 11(9): 0163645.
- 41.Bao M-H, Xiao Y, Zhang Q-S, Luo H-Q, Luo J et al. (2015) Meta-analysis of miR-146a polymorphisms association with coronary artery diseases and ischemic stroke.” International journal of molecular sciences. 16(7), 14305-14317.
- 42.Y J Jeon, O J Kim, S Y Kim, S H Oh, Oh D et al. (2013) Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk.” Arteriosclerosis, thrombosis, and vascular biology. 33(2), 420-430.
- 43.Liu G, Friggeri A, Yang Y, Park Y-J, Tsuruta Y et al. (2009) miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses.”. Proceedings of the National Academy of Sciences 106(37), 15819-15824.
- 44.Chen Y, Song Y, Huang J, Qu M, Zhang Y et al. (2017) increased circulating exosomal mirna-223 is associated with acute ischemic stroke.” Frontiers in. , Neurology 8.
- 45.Ding X-Q, Ge P-C, Liu Z, Jia H, Chen X et al. (2014) Interaction between microRNA expression and classical risk factors in the risk of coronary heart disease.” Scientific reports 5:. 14925-14925.
- 46.K S Tan, Armugam A, Sepramaniam S, K Y Lim, K D Setyowati et al. (2009) Expression profile of MicroRNAs in young stroke patients.”. , PloS one 4(11), 7689.
- 47.Zhang X, Fernández-Hernando C. (2017) . miR-33 Regulation of Adaptive Fibrotic Response in Cardiac Remodeling, Am Heart Assoc .
- 48.Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S et al. (2012) MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE−/− mice.”. , Journal of the American Heart Association 1(6), 003376.
- 49.X J Wei, Han M, F Y Yang, G C Wei, Z G Liang et al. (2015) Biological significance of miR-126 expression in atrial fibrillation and heart failure.”. , Brazilian Journal of Medical and Biological Research 48(11), 983-989.
- 50.Chen J, Cui C, Yang X, Xu J, Venkat P et al. (2017) MiR-126 Affects Brain-Heart Interaction after Cerebral Ischemic Stroke.” Transl Stroke Res.
- 51.Penzkofer D, Bonauer A, Fischer A, Tups A, R P Brandes et al. (2014) Phenotypic characterization of miR-92a−/− mice reveals an important function of miR-92a in skeletal development.”. , PLoS One 9(6), 101153.
- 52.H A Bazan, S A Hatfield, C B O’Malley, A J Brooks, Lightell D et al. (2015) Acute loss of miR-221 and miR-222 in the atherosclerotic plaque shoulder accompanies plaque rupture.”. , Stroke 46(11), 3285-3287.
- 53.Tsai P-C, Liao Y-C, Wang Y-S, Lin H-F, Lin R-T et al. (2013) Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease.”. , Journal of vascular research 50(4), 346-354.
- 54.Qian L, Laake L W Van, Huang Y, Liu S, M F Wendland et al. (2011) miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes.”. , Journal of Experimental Medicine: 20101547.
- 55.Maegdefessel L, J M Spin, Raaz U, S M Eken, Toh R et al. (2014) miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development.” Nature communications 5.
- 56.Yin K-J, Hamblin M, Y Eugene Chen. (2015) Angiogenesis-regulating microRNAs and ischemic stroke.” Current vascular pharmacology. 13(3), 352-365.
- 57.Deng L, F J Blanco, Stevens H, Lu R, Caudrillier A et al. (2015) miR-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension.” Circulation research:CIRCRESAHA. 115-306806.
- 58.Wei Y-S, Xiang Y, Liao P-H, Wang J-L, Peng Y-F. (2016) An rs4705342 T> C polymorphism in the promoter of miR-143/145 is associated with a decreased risk of ischemic stroke.”. , Scientific Reports 6.
- 59.Okada M, Kim H, Ashraf M, Kawabe J-i, Hasebe N. (2014) . Abrogation of miR-195 Improves Function in Aged Heart by Preventing Telomere Shortening and Mitochondrial Dysfunction.” Circulation 130(Suppl 2): 13060-13060.
- 60.Soeki T, Yamaguchi K, Niki T, Uematsu E, Bando S et al. (2015) Plasma microRNA-100 is associated with coronary plaque vulnerability.”. , Circulation Journal 79(2), 413-418.
- 61.Wang Z-h, Zhang Q-s, Duan Y-l, Zhang J-l, Li G-f et al. (2015) TGF-β induced miR-132 enhances the activation of TGF-β signaling through inhibiting SMAD7 expression in glioma cells.” Biochemical and biophysical research communications. 463(3), 187-192.
- 62.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W et al. (2002) Identification of tissue-specific microRNAs from mouse.”. , Current biology 12(9), 735-739.
- 63.Zhang C. (2008) MicroRNAs: role in cardiovascular biology and disease.”. , Clinical Science 114(12), 699-706.
- 64.Silvestri P, C Di Russo, Rigattieri S, Fedele S, Todaro D et al. (2009) MicroRNAs and ischemic heart disease: towards a better comprehension of pathogenesis, new diagnostic tools and new therapeutic targets.” Recent patents on cardiovascular drug discovery 4(2):. 109-118.
- 65.Cushing L, Kuang P P, Qian J, Shao F, Wu J et al. (2011) miR-29 is a major regulator of genes associated with pulmonary fibrosis.” American journal of respiratory cell and molecular biology 45(2):. 287-294.
- 66.Cheng Y, Zhang C. (2010) MicroRNA-21 in cardiovascular disease.”. , Journal of cardiovascular translational research 3(3), 251-255.
- 67.Cao W, Shi P, Ge J-J. (2017) miR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway.” BMC cardiovascular disorders 17(1):. 88.
- 68.Qin Y, Yu Y, Dong H, Bian X, Guo X et al. (2012) MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis.”. , Int J Med Sci 9(6), 413-423.
- 69.Wang J, Song Y, Zhang Y, Xiao H, Sun Q et al. (2012) Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice.”. , Cell research 22(3), 516-527.
- 70.E Van Rooij, L B Sutherland, Liu N, A H Williams, McAnally J et al. (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure.”. Proceedings of the National Academy of Sciences 103(48), 18255-18260.
- 71.S P Romaine, Tomaszewski M, Condorelli G, N J Samani. (2015) MicroRNAs in cardiovascular disease: an introduction for clinicians.”. , Heart 101(12), 921-928.
- 72.Olivieri F, Antonicelli R, Lorenzi M, D’Alessandra Y, Lazzarini R et al. (2013) Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction.”. , International journal of cardiology 167(2), 531-536.
- 73.Mizuno K, Mataki H, Seki N, Kumamoto T, Kamikawaji K et al. (2017) MicroRNAs in non-small cell lung cancer and idiopathic pulmonary fibrosis.”. , Journal of Human Genetics 62(1), 57-65.
- 74.Zhu K, Liu D, Lai H, Li J, Wang C. (2016) Developing miRNA therapeutics for cardiac repair in ischemic heart disease.”. , Journal of Thoracic Disease 8(9), 918.
- 75.Pan Z, Sun X, Shan H, Wang N, Wang J et al. (2012) MiR-101 inhibited post-infarct cardiac fibrosis and improved left ventricular compliance via FOS/TGFβ1 pathway.” Circulation:. CIRCULATIONAHA. 112.094524
- 76.Tao H, Yang J-J, Hu W, Shi K-H, Deng Z-Y et al. (2016) Noncoding RNA as regulators of cardiac fibrosis: current insight and the road ahead.”. , Pflügers Archiv-European Journal of Physiology 468(6), 1103-1111.
- 77.Isik M, T K Blackwell, Berezikov E. (2016) MicroRNA mir-34 provides robustness to environmental stress response via the DAF-16 network in C. , elegans.” Scientific Reports 6.
- 78.Al-Zikri P N H, Taher M, Susanti D, M F Rezali, Read R W et al. (2014) Cytotoxic tirucallane triterpenes from the stem of Luvunga scandens.”. , Revista Brasileira de Farmacognosia 24(5), 561-564.
- 79.Zacchigna S, Giacca M. (2014) Extra-and intracellular factors regulating cardiomyocyte proliferation in postnatal life.”. , Cardiovascular research 102(2), 312-320.
- 80.H S Cheng, Sivachandran N, Lau A, Boudreau E, J L Zhao et al. (2013) MicroRNA‐146 represses endothelial activation by inhibiting pro‐inflammatory pathways.’. , EMBO molecular medicine 5(7), 1017-1034.
- 81.Li K, Ching D, F S Luk, Raffai R L. (2015) . Apolipoprotein E Enhances MicroRNA-146a in Monocytes and Macrophages to Suppress Nuclear Factor-κB–Driven Inflammation and AtherosclerosisNovelty and Significance.” Circulation research 117(1): 1-11.
- 82.Xing G, Luo Z, Zhong C, Pan X, Xu X. (2016) Influence of miR-155 on Cell Apoptosis in Rats with Ischemic Stroke: Role of the Ras Homolog Enriched in. , Brain (Rheb)/mTOR Pathway.” Medical Science Monitor 22, 5141-5153.
- 83.Ling H, Fabbri M, G A Calin. (2013) MicroRNAs and other non-coding RNAs as targets for anticancer drug development.” Nature reviews Drug discovery. 12(11), 847-865.
- 84.E A Orellana, A L Kasinski. (2015) MicroRNAs in cancer: a historical perspective on the path from discovery to therapy.”. , Cancers 7(3), 1388-1405.
- 85.D J Hearse, Bolli R. (1992) Reperfusion induced injury: manifestations, mechanisms, and clinical relevance.”. , Cardiovascular research 26(2), 101-108.
- 86.M A Song, A N Paradis, M S Gay, Shin J, Zhang L. (2015) Differential expression of microRNAs in ischemic heart disease.’ Drug discovery today. 20(2), 223-235.
Cited by (1)
- 1.Bai Xiaodan, Hua Shengyu, Zhang Junping, Xu Shixin, 2019, The MicroRNA Family Both in Normal Development and in Different Diseases: The miR-17-92 Cluster, BioMed Research International, 2019(), 1, 10.1155/2019/9450240
