Abstract
Pulmonary hypertension (PH) has become an increasingly recognized complication in sickle cell anaemia (SCA) and is a major cause of morbidity and mortality. Though the burden of SCA in sub-Saharan Africa is high, there is paucity of data on SCA-associated PH with little or no attention given to it in routine patient care. The current study therefore sought to determine the prevalence of PH and its associated risk factors among adult patients with SCA. This was a cross-sectional study involving 76 clinically stable, hydroxyurea-naive participants. We obtained socio-demographic and clinical history. Measurement of Tricuspid Regurgitant jet Velocity (TRV) was obtained via transthoracic echocardiography and lung function was assessed using spirometry and pulse oximetry. Other investigations were complete blood counts, free plasma haemoglobin, serum urea and creatinine. Twenty-five (32.9%) of study participants had elevated TRV (≥ 2.5m/s) on Doppler echocardiography, which was suggestive of raised pulmonary artery systolic pressure. There were significant associations between elevated TRV and steady-state haemoglobin (p < 0.001), blood urea level (p = 0.030), presence of chronic leg ulcers (p = 0.043) and oxygen saturation (p < 0.001) and these may be identifiable and modifiable risk factors for selective screening with echocardiography in a resource poor setting.
Author Contributions
Academic Editor: Krzysztof Roszkowski, Nicolaus Copernicus University, PL. Department of Oncology, Radiotherapy and Gynecologic Oncology.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Jane S. Afriyie-Mensah, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Pulmonary involvement in sickle cell disease (SCD) has been shown to be a major factor affecting morbidity and mortality, being recognized also as an important determinant of survival 1,2.This has become apparent in recent times as increased numbers of SCD patients reach adulthood due to improved survival strategies in childhood 3. The lungs are common sites of hypoxic and ischaemic injury, emboli from marrow infarcts/fat necrosis with increased propensity to developing pneumonias 4. Chronic lung complications in sickle cell disease describes a state of permanent damage to the lung parenchyma and vasculature. Manifestations include interstitial abnormalities/fibrosis, restrictive lung disease and pulmonary hypertension in its severe form 5. Emerging evidence have consistently shown that pulmonary hypertension (PH) complicated by right heart failure is a major cause of mortality among adult patients with disease, particularly those with homozygous disease, sickle cell anaemia (SCA)6,7,8. Pulmonary hypertension is usually asymptomatic in the early stages but may be characterized by dyspnoea on exertion, hypoxaemia, progressive worsening of pulmonary function tests (PFTs) and right heart failure when severe 9. Even in advanced stages, the elevation in pulmonary artery systolic pressures (PASP) has been shown to be mild to moderate in sickle cell patients compared to those with idiopathic or scleroderma associated PH, yet the former presents with equivalent symptoms of severe dyspnoea, exercise limitation, hypoxaemia and progressive right heart failure 10. This spells out the need for screening and early identification of elevations in PASP using non-invasive means such as trans-thoracic echocardiography (ECHO) instead of invasive right heart catheterization (RHC), which is the diagnostic procedure. This is a validated tool for detecting early rise in pumonary artery pressure, with PH being more likely if the tricuspid regurgitant jet velocity (TRV) is ≥ 2.5m/s. The prevalence of elevated TRV using echocardiography is estimated to be 25-30% among SCA patients and 10-25% in those with genotype SC 6,8,11. However, studies have reported that only about 6-11% of patients with TRV ≥ 2.5m/s on ECHO have confirmed pulmonary hypertension on right heart catheterization 12,13. Notwithstanding, elevated TRV ≥ 2.5m/s in sickle cell patients has been significantly associated with decreased exercise tolerance and increased mortality estimated to be about 40% at 40 months of follow-up and is therefore considered an independent risk factor for death among these patients 6,14,15. According to the official American Thoracic Society (ATS) practice guideline 16, mortality risk of sickle cell patients can be determined non-invasively by measurement of TRV via doppler echocardiography. The current study sought to determine wether elevated TRV is an identifiable risk factor among our SCA cohort and if present determine associated clinical and laboratory risk factors.
Methods
Study Design
This was a analytical cross-sectional study involving 80 hydroxyurea-naive adult SCA participants, 18yrs and above, who were in their steady state and consented to participate in the study. The study was carried out within the period of March – April, 2014.
Study Site
This study was conducted at the Ghana Institute of Clinical Genetics which renders outpatient and day care services with referrals from other healthcare facilities all over Ghana. It has the largest adolescent and adult sickle cell clinic in Ghana, with over 25,000 registered sickle cell patients in its database. Averagely the clinic attends to 40 patients daily.
Data Collection
Sickle cell anaemia patients with clear documentation of results of electrophoresis in their clinic notes were selected by systematic random sampling using the daily clinic attendence list and screened for eligibility. Four (4) patients were excluded from analysis due to our inability to measure the TRV jet. Patients needed to be in their steady state as acute crisis could cause temporary rise in TRV values and lower lung volumes on spirometry 17. A structured and pre-tested questionnaire was used to obtain socio-demographic and clinical information on all participants. A modified version of St. George’s Respiratory questionnaire was used to screen participants for chronic chest diseases and excluded if present. Blood pressure, pulse rate and oxygen saturation were recorded at rest. A general and cardio-respiratory examination were performed on all participants to further exclude those with respiratory and cardiovascular diseases that are capable of influencing ECHO and spirometry results of the stable participants. Blood samples for complete blood count, blood urea, creatinine and free plasma haemoglobin were taken.Spirometry was performed according to ATS protocol using SCHILLER SPIROVIT SP-1 (Schiller-AG,Switzerland) 18. Forced vital capacity (FVC) and forced expiratory volume per second (FEV1) were measured.
Trans-thoracic doppler echocardiography was performed for all participants using a Toshiba brand ECHO machine, Aplio 300 3MHz transducer and in accordance with the Ameican echocardiography Soceity guidelines19. Three different measurements were taken at rest and the average recorded as the mean TRV. where values ≥ 2.5m/s were classified as having elevated TRV and those < 2.5m/s as normal. A value of 2.5m/s-2.9m/s was suggestive of mild rise in PASP and values of 3.0m/s or more regarded as moderate to severe rise.
Free plasma haemoglobin was determined using the solid phase-phase sandwich ELISA technique. Optimization was pre-determined prior to running the test sample. The normal reference range of free plasma Hb in non-sickle cell patients is 10 - 40µg/ml 20. Free plasma haemoglobin values above 40 ug/ml was regarded as abnormal and suggestive of ongoing intravascular haemolysis.
Data Analysis and Ethical consideration
All information from the questionnaire were captured and cleaned and later exported into Statistical Package for Social Sceinces (SPSS) version 22 for data analysis. The Laboratory parameters, TRV and spirometry measurements (FVC and FEV1) were summarized by means and standard deviations. Spearmans correlation was used to establish association between spirometry, laboratory and doppler echocardiogram findings. Linear regression and multivariate analysis were used to examine significant factors influencing TRV outcomes among the sickle cell patients.
Ethical Approval was obtained from the Ethical and Protocol Review Committee of the College of Health science, University of Ghana. (Protocol identification number- CHS-Et/M.10-P3.9/2013-2014). Clearance was also received from the Head of the GICG where the review was conducted.
Results
Demographic and Clinical Data
The mean age for the SCA subjects was 33.6±11.1 years with a range of 18-63 years and majority (60.5%) were females. The mean height and weight were 164.3cm and 59.0kg respectively, with a mean BMI of 21.8kg/m2(Table 1). Clinical history showed that 6(7.9%) of the SCA subjects had hypertension, 1(1.3%) had diabetes and 1(1.3%) had chronic kidney disease (Table 2). Fifty-three (69.7%) of the participants had a history of previous blood transfusion and 28 (36.8%) had a history of chronic leg ulcer (Table 2). Nine (25.7%) out of the 30 male participants had a previous history of priapism, mostly during the adolescent period. Sixty-three (82.9%) had between 0 – 2 and 13 (17.1%) had 3 or more episodes of sickle cell crisis in the past year, mostly reported as bone pain (Table 2).
Table 1. Descriptive summary of Anthropometric, Spirometry and laboratory measurements of SCA participants.| Anthropometry results | Mean | Measured range | Std. Deviation |
|---|---|---|---|
| Weight (kg) | 59.0 | 38.0-117.5 | 13.2 |
| Height (cm) | 164.3 | 135.0-182.5 | 8.2 |
| BMI (kg/m2) | 21.8 | 15.8-40.2 | 4.2 |
| Age (years) | 33.8 | 18.0-63.0 | 11.1 |
| Spirometry results | |||
| FVC % Predicted | 72.6 | 22.2 | |
| FEV1 % Predicted | 65.3 | 20.6 | |
| % Ratio (FEV1/FVC) | 75.6 | 9.6 | |
| Laboratory results | |||
| Heamoglobin (g/dl) | 8.06 | 4.9- 11.2 | 1.6 |
| Haematocrit (%) | 24.86 | 14.2- 83.0 | 8.6 |
| MCV (fl) | 83.27 | 78.0-105.0 | 11.6 |
| MCH (pg) | 28.53 | 16.3-36.5 | 4.3 |
| Total WBC (109/l) | 10.07 | 3.4-26.0 | 3.6 |
| Platelet Count (109/l) | 341.36 | 142.0-873.0 | 109.2 |
| Free Plasma Hb (ug/ml) | 105.70 | 8.2-471.1 | 53.4 |
| Urea (mmol/l) | 3.48 | 1.6-10.7 | 1.6 |
| Creatinine (mmol/l) | 55.85 | 21.0-118.0 | 20.0 |
| Clinical Parameter | Frequency (n) | Percent (%) |
|---|---|---|
| Hypertension | 6 | 7.9 |
| Diabetes | 1 | 1.3 |
| CKD | 1 | 1.3 |
| History of Leg Ulcer (n=76) | 28 | 36.2 |
| History of Blood Transfusion (n=76) | 53 | 69.7 |
| History of Priapism (n=30) | 9 | 25.7 |
| Frequency of Sickle Cell Crises | ||
| 1 | 26 | 34.2 |
| 2 | 11 | 14.5 |
| 3 | 6 | 7.9 |
| >3 | 7 | 9.2 |
| None | 26 | 34.2 |
| Total | 76 | 100 |
Laboratory Results
From Table 1, the mean steady state haemoglobin (Hb) was 8.06 ± 1.59g/dl with a range of 4.9 – 11.2 g/dl, a mean MCV of 83.3 ± 11.6fl and MCH of 28.5 ± 4.3 pg respectively.The mean total white cell count (WBC) was elevated 10.1 ± 3.6 x 109/l with a range of 3.4 – 26 x109/l and that of the total platelet count was 341.4 ± 109.2 x109, with a wide range of143-873 x 109/l. Mean blood urea was, 3.5 ± 1.6 mmols/l and that of serum creatinine was 55.6 ± 20.0 mmols/l. The mean value of free plasma haemoglobin was 105.7± 53.4µg/ml with a range of 8.2- 471.1µg/ml. Sixty-six (90.4%) of the SCA patients had intravascular haemolysis (free plasma Hb > 40ug/ml).
Echocardiogram and TRV
Approximately a third of the SCA participants, 25(32.9%) had elevated TRV ( ≥ 2.5m/s) with 19(76%) of them having mild elevation in TRV (2.5m/s - 2.9m/s) and 6(24.0%) with moderate-severe elevation (≥ 3.0m/s). There was no significant difference between participants with elevated TRV and those with normal TRV with regards to age, BMI and frequency of sickling crisis in a year (p > 0.05). There was however a significant association between elevated TRV and history of chronic/recurrent leg ulcers (p=0.043). The mean haemoglobin level was significantly different between those with elevated TRV and those without (6.9g/dl and 8.6g/dl respectively, p < 0.001), (Table 3). Further analysis showed that among participants with elevated TRV, those with values ≥ 3.0m/s had a significantly lower mean Hb of 5.8g/dl compared to 7.3g/dl in those with values between 2.5 – 2.9m/s (p=0.023). A significant inverse correlation was also observed between steady state Hb and TRV (R= -0.456, p=0.001, Figure 1) as well as with free plasma Hb (R = - 0.104, p = 0.039).The positive correlation between TRV and free plasma Hb noted was however not statistically significant (R=0.075, p=0.534). Blood urea was significantly higher in the group with elevated TRV than in the normal TRV group (p=0.030) but there were no significant differences observed between the two groups with regards to total WBC count, platelet count, free plasma hemoglobin and creatinine level, p > 0.005 (Table 3).
Figure 1.Correlation Between Trv and Haemoglobin.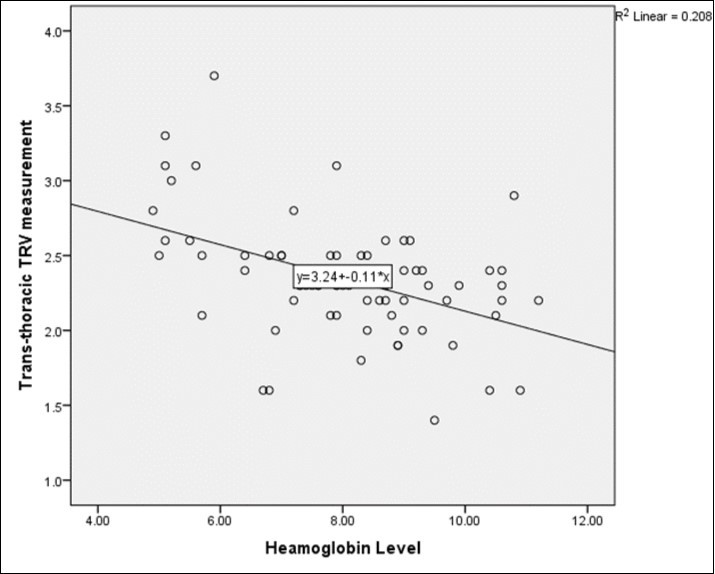
| TRV (m/s) | Hb (g/dl) | Hct | MCV | MCH | Total WBC (x109/l) | Platelet Count(x109/l) | Plasma Haemoglobin(ug/ml) | Urea(mmol/l) | Creatinine(mmol/l) | |
| Normal | Mean | 8.6 | 25.7 | 83.9 | 28.4 | 10.0 | 341.8 | 113.0 | 3.2 | 52.8 |
| Std. | 1.3 | 4.2 | 8.3 | 3.9 | 3.3 | 115.0 | 56.1 | 1.0 | 14.0 | |
| Elevated | Mean | 6.9 | 23.0 | 82.0 | 28.8 | 10.3 | 362.6 | 117.3 | 4.1 | 61.9 |
| Std. | 1.6 | 13.8 | 16.6 | 5.1 | 4.3 | 144.8 | 102.9 | 2.3 | 27.7 | |
| P-value | 0.001* | 0.194 | 0.503 | 0.682 | 0.762 | 0.506 | 0.820 | 0.030* | 0.088 | |
Spirometry
With regards to lung function, there was a significant difference in the mean oxygen saturation of participants with elevated TRV and those without (92.0% and 94.0% respectively, p = < 0.001). Spirometry measurements of participants showed a mean FVC% predicted of 72.6 and a mean FEV1% predicted of 65.3 with a mean FEV1/FVC ratio of 75.6. There were no significant differences in the FEV1 and FVC percentage predicted values of participants with elevated TRV and those without, p>0.05.
Discussion
Pulmonary hypertension (PH) complicated by right heart failure has emerged as an independent risk factor for morbidity and mortality among SCD patients, especially in those with homozygous disease. Assessment of TRV via trans-thoracic echocardiography has been shown to predict the presence of PH in SCD patients and has prognostic significance. Elevations in TRV ≥ 2.5m/s has been found to be associated with increased risk of mortality with a reported prevalence of about 25-30% among SCA patients 6,7,8. Moderate to severe elevation in TRV (≥ 3.0m/s) is associated with a higher risk of mortality and an increased probability of definite diagnosis of pulmonary hypertension on RHC 7,12,21. In the current study, 25(32.9%) had elevated TRV, ≥ 2.5m/s. Among these, the majority(76%) had mild TRV elevation of between 2.5m/s - 2.9m/s. Our prevalence is similar to the 32% reported by Gladwin et al, 2004, in which the mean age of the SCA participants (36 ± 12 years) is comparable to that of our cohort. A study by Aliyu et al found a prevalence of 25% among a cohort of younger Nigerian SCA patients with mean age 22 ± 8 years 11. There is some suggestion of higher TRV values with increasing age of SCA patients and this could have accounted for the lower prevalence in the Nigerian study 22. Our study however, showed a positive but non-significant correlation between age and TRV measurements (R=0.147, p=0.205). The presence of elevated TRV in about a third of the participants revealed that PH as a complication of SCA may not be a rare occurrence among our SCA cohort, though this has not been previously studied. This result gives the realization that as recommended by the ATS adhoc committee on pulmonary hypertension, our SCA patients should also be periodically screened for early detection of PH 16.
The current study showed a low mean steady state haemoglobin, raised total WBC count, among the SCA subjects. These results are consistent with the common haematological profile of patients with SCA 20. The baseline steady-state haemoglobin in sickle cell patients has been shown to be within the range of 6 – 9g/dl and this study similarly reported a mean of 8.06 ± 1.59g/dl which was largely normocytic, normochromic 20,23. Few participants had microcytic hypochromic anaemia which may have been due to coexisting iron deficiency anaemia; however iron status of the participants was not assessed. The proportion of participants with intravascular haemolysis was very high (90.41%) and this was not surprising as patients with SCA are known to have more severe chronic haemolysis 20. Our analysis further showed a significant negative correlation between free plasma Hb (a marker of intravascular haemolysis) and steady-state Hb level (R = - 0.104, P=0.039). This suggests that intravascular haemolysis, may have been the major cause of the low steady state Hb observed.
Previous studies have shown that markers of intravascular haemolysis such as lactate dehydrogenase (LDH) and free plasma Hb, are significantly associated with vascular complications of sickle cell disease including PH, strokes, priapism, leg ulcers and renal failure 5,15,24. There are also reported associations between the presence of PH, raised levels of free plasma Hb and Nitric oxide (NO) consumption within the vascular endothelium 25, 26, 27. These studies have proposed that endothelial NO, which maintains vascular intergrity and vasodilatory function, are depleted by the release of toxic products such as free plasma Hb and arginase-1 from lysed red cells. Therefore, chronic intravascular haemolysis as occurs in sickle cell patients, leads to the depletion of haptoglobin and haemopexin, which are natural scavengers of free plasma Hb 28. The blood level of free plasma Hb subsequently rises appreciably and exerts its effect by mopping up considerable amounts of Nitric Oxide (NO) from witihin the vascular endothelial cells. In addition, arginase 1, breaks down arginine, an important substrate for intracellular NO production. Both processes concurrently result in endothelial NO deficiency and the long-term effect is the development of sickle cell diease associated vasculopathy, a precursor to developing PH 25,26,29.
The current study similarly found a significant inverse correlation between the steady state Hb and TRV as well as between steady state Hb and free plasma Hb 6,30. There was however no significant inverse correlation between TRV and free plasma Hb. This finding question the proposed direct role of free plasma Hb induced NO deficiency in the aetiology of PH among SCA patients. In support of this, a study by Machado et al, found no evidence of reduction in the mean pulmonary artery systolic pressure or TRV measurements when SCA patients with RHC confirmed PH were treated with 5’ phosphodiesterase inhibitors, a drug that increases bioavailability of NO within endothelial cells 31. We therefore suggest that the additive effect from other products of chronic haemolysis released from the lysed red cells, including endothelial adhesion factors, procoagulant factors, phosphatidyl serine, could be contributing to the pulmonary vascular endothelial damage, hence the absence of a direct association between TRV and free plasma haemoglobin as observed in other studies 32,33.
The effect of chronic hypoxia on the integrity of pulmonary vasculature has been shown to be an important cause of PH in SCA 34. Some studies have shown that chronic anaemia in SCA is associated with chronic up-regulation of the hypoxic response through activation of hypoxia inducible factor (HIF-α) with attendant damage to the pulmonary vascular endothelium 35. The strength of association between low steady state hb and TRV is emphasized by the evidence that chronic scheduled transfusions reduce most complications of SCD such as strokes, acute chest syndrome (ACS) and vaso-occlusive crisis (VOC) 36. These benefits are achieved through reduction in the burden of sickled RBCs and the rate of haemolysis. Lezcano et al, 2006, also showed that chronic transfusions reduce free plasma Hb with subsequent lowering of TRV among SCD patients studied 37. These findings together with that from the current study, show that improving the most significant risk factor, low steady-state haemoglobin, is key in preventing/reducing the occurrence of vascular complications of SCA patients such as PH.
Assessing the association with other vascular complications in SCA, the current study found a significant relationship between TRV and chronic/recurrent leg ulcers (p = 0.043) but not with priapism (p=0.253). Although we also found a significant association between serum urea and TRV, which compares with findings in previous studies, the former is not a measure of kidney function and measurement of urine microalbumin would have been a better marker of early renal involvement 6,11,38,39.
Frequency of sickling crisis, which is considered a marker of disease severity among SCA patients was not statistically different between the groups with elevated TRV and those without 39. The current study showed significantly lower mean oxygen saturation in patients with elevated TRV compared to those with normal values (92.0% and 94.0% respectively, p < 0.001). As similarly observed in previous studies, this could reflect the role of hypoxia in the development of PH 6,39.
In conclusion, steady-state Hb is a significant risk factor for elevated TRV in SCA, with increased risk when steady-state Hb is 6.9g/dl or less. In view of this finding, we recommend that steady-state Hb could be used to selectively identify SCA patients who need screening for PH, particularly in low resource settings. Also, measures to reduce or prevent hyperhaemolysis in SCA patients such as hydroxyurea and chronic scheduled transfusions should be considered in our setting, although the latter may not be immediately feasible in low income countries.
Acknowledgements
We are grateful to all sickle cell patients who participated in this study, the staff of the Ghana institute of clinical genetics, Mr Emmanuel Allotey and our research assistant Mr Ernest Kwarteng for helping with data collection.
Some findings of this study were presented at ASH 2017 and the abstract published in Blood 2017 130:2261.
References
- 1.Gray A, E N Anionwu, S C Davies, Brozovic M. (1991) Patterns of mortality in sickle cell disease in the United Kingdom. , J Clin Pathol 44(6), 459-63.
- 2.A N Thomas, Pattison C, G R Serjeant. (1982) Causes of death in sickle-cell disease in Jamaica. , BMJ 285(6342).
- 3.O S Platt, D J Brambilla, W F Rosse. (1994) Mortality In Sickle Cell Disease - Life Expectancy and Risk Factors for Early Death. , N Engl J Med 330(23), 1639-44.
- 4.S H Embury, R P Hebbel, Mohandas N, M H Steinberg. (1995) Sickle Cell Anaemia: basic principles and clinical practice.New York:Raven Pres. 685-690.
- 5.Powars D, J A Weidman, Odom-Maryon T, J C Niland. (1988) Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. , Medicine (Baltimore) 67, 66-76.
- 6.M T Gladwin, Sachdev V, M L Jison. (2004) Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. , N Engl J Med 350(9), 886-95.
- 7.Mehari A, M T Gladwin, Tian X. (2012) Mortality in adults with sickle cell disease and pulmonary hypertension. , JAMA 307(12), 1254-6.
- 8.Castro O, Hoque M, B D. (2003) Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. , Blood 101(4), 1257-61.
- 9.D R Powars, L S Chan, Hiti A. (2005) Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. , Medicine (Baltimore) 84(6), 363-76.
- 10.Anthi A, R F Machado, M L Jison. (2007) Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. , Am J Respir Crit Care 175(12), 1272-9.
- 11.Z Y Aliyu, Gordeuk V, Sachdev V. (2008) Prevalence and risk factors for pulmonary artery systolic hypertension among sickle cell disease patients in Nigeria. , Am J Hematol 83(6), 485-90.
- 12.Parent F, Bachir D, Inamo J. (2011) A hemodynamic study of pulmonary hypertension in sickle cell disease. , N Engl J Med 365(1), 44-53.
- 13.G H Fonseca, Souza R, V M Salemi. (2012) Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. , Eur Respir J 39(1), 112-8.
- 14.K I Ataga, C G Moore, Jones S. (2006) Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. , Br J Haematol 134(1), 109-15.
- 15.LM De Castro, Jonassaint J C, Graham F L. (2008) Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. , Am J Hematol 83(1), 19-25.
- 16.E S Klings, R F Machado, R J Barst. (2014) American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease. An Official American Thoracic Society Clinical Practice Guideline: Diagnosis, Risk Stratification, and Management of Pulmonary Hypertension of Sickle Cell Disease. , Am J Respir Crit Care 189(6), 727-40.
- 17.R F Machado, M A Kyle, Martyr S. (2007) Sverity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. , Br J haematol 136, 319-325.
- 18.Standardization of Spirometry,1994 Update.(1995)American Thoracic Society. , Am J Respir Crit Care Med 152(3), 1107-36.
- 19.Berger M, Haimowitz A, A V Tosh. (1985) Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. , J Am Coll Cardiol 6(2), 359-365.
- 20.M T Gladwin, Vinchinsky E. (2008) Pulmonary complications of sickle cell disease. , N Engl J Med 359, 2254-2265.
- 21.M K Sandhu, Cohen A. (2015) Aging in sickle cell diseases: Co-moorbidities and new issues in management. , International Journal of hemoglobin research 39(4), 221-224.
- 22.Akinbami A, Adedoyin D, Adewumi A. (2012) Steady state hemoglobin concentration and packed cell volume in homozygous sickle cell disease patients in Lagos. , Nigeria. Casp J.Intern Med 3(2), 405-9.
- 23.G A Barabino, M O Platt, D K Kaul. (2010) Sickle cell biomechanics. , Annu Rev Biomed Eng 12, 345-367.
- 24.Schaer D J, Buehler P W, Alayash A I. (2013) Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. , Blood 121(8), 1276-84.
- 25.C D Reiter, Wang X, J E Tanus-Santos. (2002) Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. , Nat Med 8(12), 1383-9.
- 26.Hebbel R. (2011) Reconstructing sickle cell disease: A data‐based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence. , Am J Hematol 86(2), 123-154.
- 27.R P, Bell L, Hillmen P, M T Gladwin. (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. , JAMA 293(13), 1653-62.
- 28.K P Potoka, M T Gladwin. (2015) Vasculopathy and pulmonary hypertension in sickle cell disease. , Am J Physiol Lung Cell Mol Physiol 308(4), 314-24.
- 29.G H Fonseca, Souza R, V M Salemi. (2012) Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. , Eur Respir J 39(1), 112-8.
- 30.R F Machado, R J Barst, N A Yovetich. (2011) walk-PHaSST Investigators and Patients. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV andlow exercise capacity. , Blood 118(4), 855-864.
- 31.A C Frei, Guo Y, D W Jones. (2008) Vascular dysfunction in a murine model of severe hemolysis. , Blood 112(2), 398-405.
- 32.Westerman M, Pizzey A, Hirschman J. (2008) Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. , Br J Haematol 142(1), 126-35.
- 33.M T Lee, Piomelli S, Granger S. (2006) Stroke Prevention Trial in Sickle Cell Anaemia (STOP): extended follow-up and final results. , Blood 108, 847-852.
- 34.N E Lezcano, Odo N, Kutlar A. (2006) Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. , Stroke 37(6), 1424-1426.
- 35.M H Steinberg. (2005) Predicting clinical severity in sickle cell anaemia. , Br J Haematol 129(4), 465-81.
- 37.L A Shimoda, G L Samenza. (2011) HIF and the Lung: role of hypoxia-inducible factors in pulmonary development and disease. , Am J Respir Crit Care Med 183(2), 152-156.
Cited by (2)
- 1.Dei-Adomakoh Yvonne A., Afriyie-Mensah Jane S., Forson Audrey, Adadey Martin, Ndanu Thomas A., et al, 2019, Lung Function Abnormalities in Sickle Cell Anaemia, Advances in Hematology, 2019(), 1, 10.1155/2019/1783240
- 2.Ghazaiean Mobin, Darvishi-Khezri Hadi, Najafi Behnam, Karami Hossein, Kosaryan Mehrnoush, et al, 2025, Global prevalence of elevated estimated pulmonary artery systolic pressure in clinically stable children and adults with sickle cell disease: A systematic review and meta-analysis, PLOS ONE, 20(2), e0318751, 10.1371/journal.pone.0318751