Diabetic Foot and Leg Ulcer & Peri-wound Neuropathy Healing Feasibility Studies
Abstract
Diabetic foot and leg ulcers represent a significant global health burden and are frequently associated with peripheral neuropathy, vascular compromise, infection, and high rates of recurrence and amputation. Standard wound care often fails to achieve healing in chronic cases due to unaddressed underlying neuropathic and vascular pathology. This feasibility study evaluated the Hemastyl™ System in patients with long-standing diabetic foot and leg ulcers that had failed standard care and, in many cases, had been diagnosed for amputation. Two prospective feasibility cohorts comprising 39 chronic infected diabetic wounds were treated with the Hemastyl™ System. Outcomes included rapid microbe reduction, high wound closure rates, subjective improvement in neuropathy-related symptoms, and avoidance of amputation in all amputation-diagnosed cases. These findings suggest that targeting neuropathy, vasculature, and microbial burden concurrently may offer a promising approach for healing complex chronic wounds in high-risk populations.
Author Contributions
Academic Editor: Sasho Stoleski, Institute of Occupational Health of R. Macedonia, WHO CC and Ga2len CC
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2026 Margaret Kalmeta.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The author has no conflicts of interest to declare.
Citation:
Introduction
Diabetic foot diseases have continued to rise over the years across the globe. Diabetic foot ulcers result from multiple risk factors, 1 including loss of protective sensation due to peripheral neuropathy in which the feet become numb against traumas, infection, ulceration, damages to deep tissues associated with neurological abnormalities, vitamin D deficiency, and varying levels of peripheral vascular disease in the lower limb. Among the major risk factors for limb-threatening diabetic foot infections, the most significant are diabetic neuropathy, hyperglycemia, impaired inflammatory responses, and peripheral arterial disease2. Diabetic foot ulcers affect roughly 15 to 25 percent of all diabetic patients over their lifetime, and once a diabetic ulcer develops, the risk of lower-limb amputation increases significantly 3. During the period 2010-2020, the global incidence of minor and major amputations due to diabetes was estimated to be 139.97 and 94.82 cases per 100,000 diabetics, respectively 4. These ulcers also carry a high recurrence rate, with studies showing that up to 40 percent of patients develop another ulcer within one year of healing 5. This data was further worsened by the impact of the recent COVID-19 due to the ensuing complexities. Moreover, unlike other wounds, Diabetic foot ulcers (DFUs) rarely respond to standard wound care procedures due to the pathophysiology of the disease.
Chronic leg ulcers, particularly venous leg ulcers (VLUs), similarly pose a major global health burden. Recent meta-analytic data report that VLUs account for an estimated 70–90% of all chronic leg ulcers 6. Despite appropriate treatment, recurrence remains a major concern: about 22% of patients experience relapse within 3 months, 57% within 12 months, and up to 78% within 3 years of initial healing 7. These high recurrence rates highlight the chronic nature of leg ulcers and the need for sustained, long-term management strategies.
Standard dressings and treatments for wounds are rarely effective when used alone on diabetic and other chronic non-healing ulcers 8. This limited efficacy is largely attributed to unaddressed peripheral neuropathy and long-standing neural and vascular damage, which interfere with normal wound healing processes.
Introducing the Hemastyl™ System
The Hemastyl™ system technology consists of Hemastyl, a bioactive xenograft in a bottle; and the PeriWound NerveStim, a neuromuscular Electrical-Stim device. The system was born in the clinical setting and has healed neuropathy (nerves and vasculature) in our Feasibility Studies and regenerated nerves, vasculature and muscle in our Diabetic Mouse Study.
The technology uniquely targets peripheral neuropathy nerve and vascular damage, which is a major driver of chronic, non-healing wounds for which there is currently no solution. By regenerating nerves and vasculature, the Hemastyl™ system directly tackles the root cause of months’ or years’ long tissue degeneration, offering a groundbreaking solution in an underserved market Figure 1.
Figure 1.The above patient was diagnosed for an amputation but did not schedule his amputation and enrolled in our feasibility study instead. His previous treatment consisted of the current standard of care which was ineffective in healing his leg and wound. Hemastyl™ System is a dual action product, healing neuropathy while eliminating harmful microbes.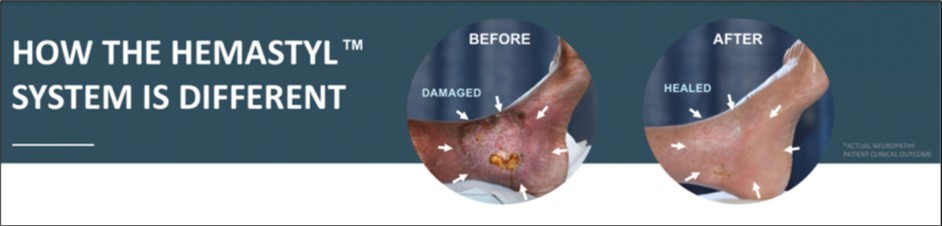
Methods
Study Design
Two prospective early-stage feasibility studies were conducted to evaluate the safety and effectiveness of the Hemastyl™ System in patients with chronic, infected diabetic foot and leg ulcers. These studies were not designed as randomized controlled trials but as feasibility research investigations.
Regulatory and Ethical Oversight
The studies were conducted in Costa Rica under local regulatory frameworks applicable to non-FDA regulated feasibility research. Approval was obtained at the facility level, including authorization from the nursing home owner, and the studies were registered with relevant governmental authorities. No formal institutional review board approval was required for this early feasibility work.
Patient Enrollment
Patients were prospectively enrolled and informed that the Hemastyl™ System represented a new treatment system. Written informed consent was obtained prior to participation.
Patient Population
A total of 39 patients with infected chronic diabetic non-healing wounds were included across two feasibility cohorts (n = 17 and n = 22). All patients had previously received standard wound care for three months or longer without progression toward healing. Wound duration ranged from approximately two to forty years. Nineteen patients had been diagnosed for potential amputation prior to enrollment.
Treatment Protocol
Treatment duration ranged from one to forty weeks depending on wound severity and tissue damage. Patients underwent clinician examinations three times per week. Microbe reduction was typically observed within one to two days following treatment initiation.
Outcome Measures
Evaluated outcomes included wound closure, microbe reduction, periwound recovery, subjective neuropathy improvement, and avoidance of amputation.
Results
Feasibility Cohort 1 (n = 17)
17 patients with Wagner grade 2–4 chronic diabetic non-healing wounds were treated with the Hemastyl. Microbe reduction occurred within one to two days, and the wound closure rate was 99 percent. Five patients reported restored warmth, four regained sensation, and six reported increased sensation. All amputations were averted Figure 2, Figure 3, Figure 4.
Figure 2.Periwound Sensory Improvement Following Treatment (n = 17 vs n = 22)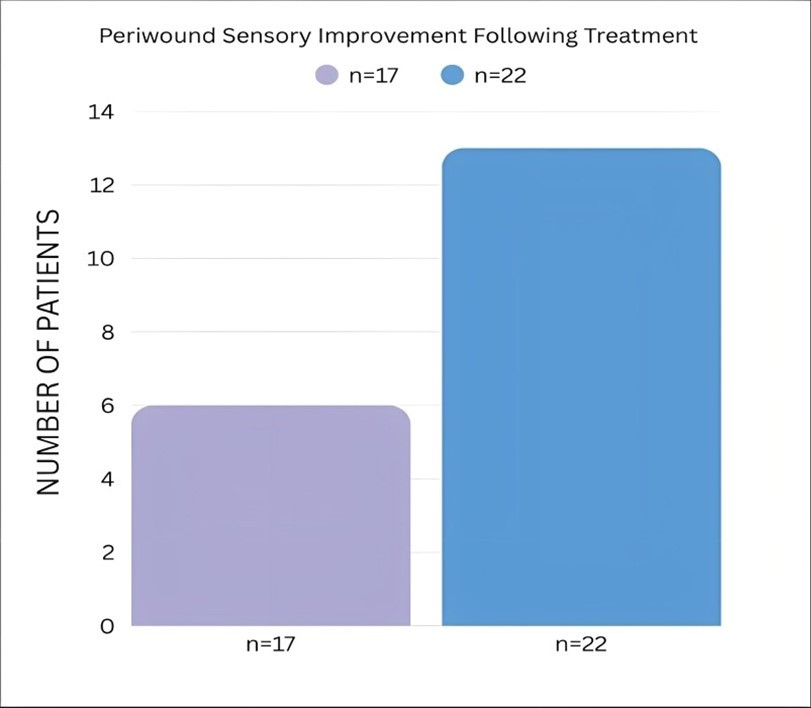
Figure 3.Partial and Full Restoration of Periwound Sensation by Cohort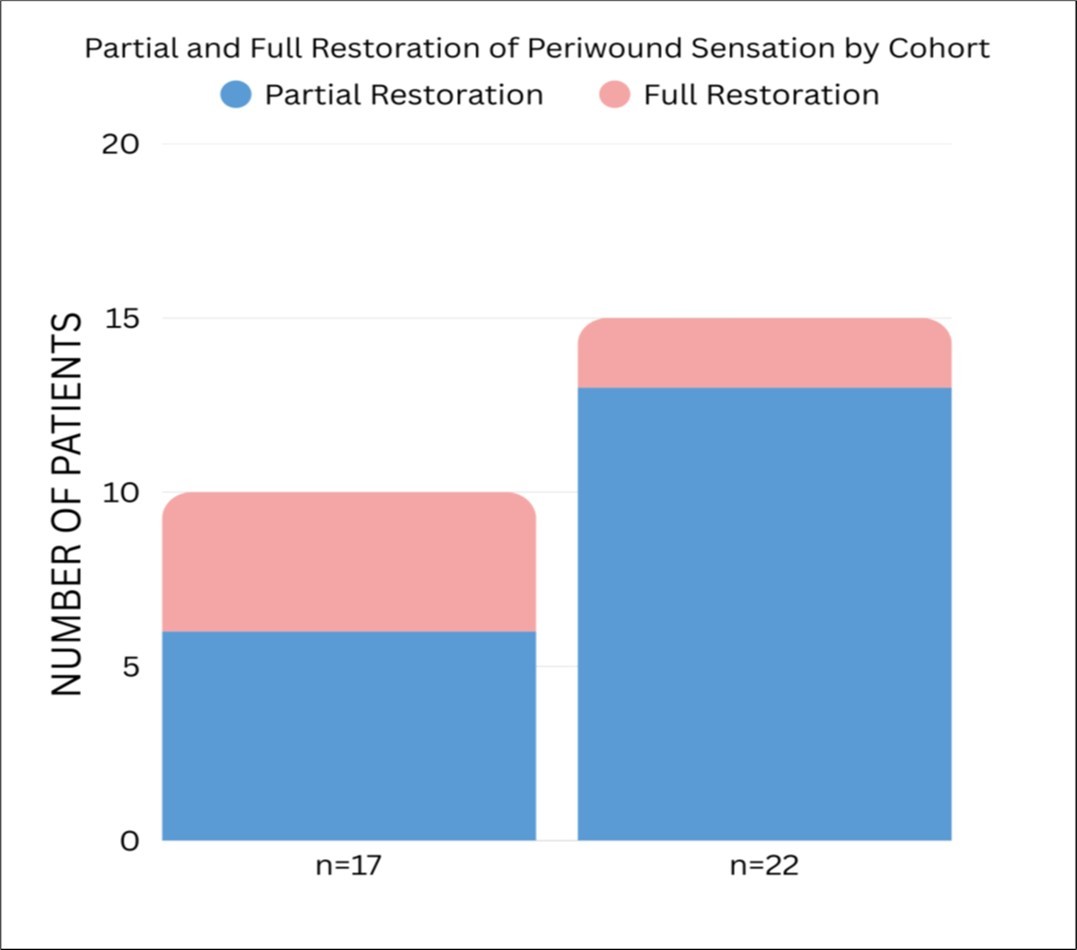
Figure 4.Patient-Reported Periwound Limb Warmth Following Treatment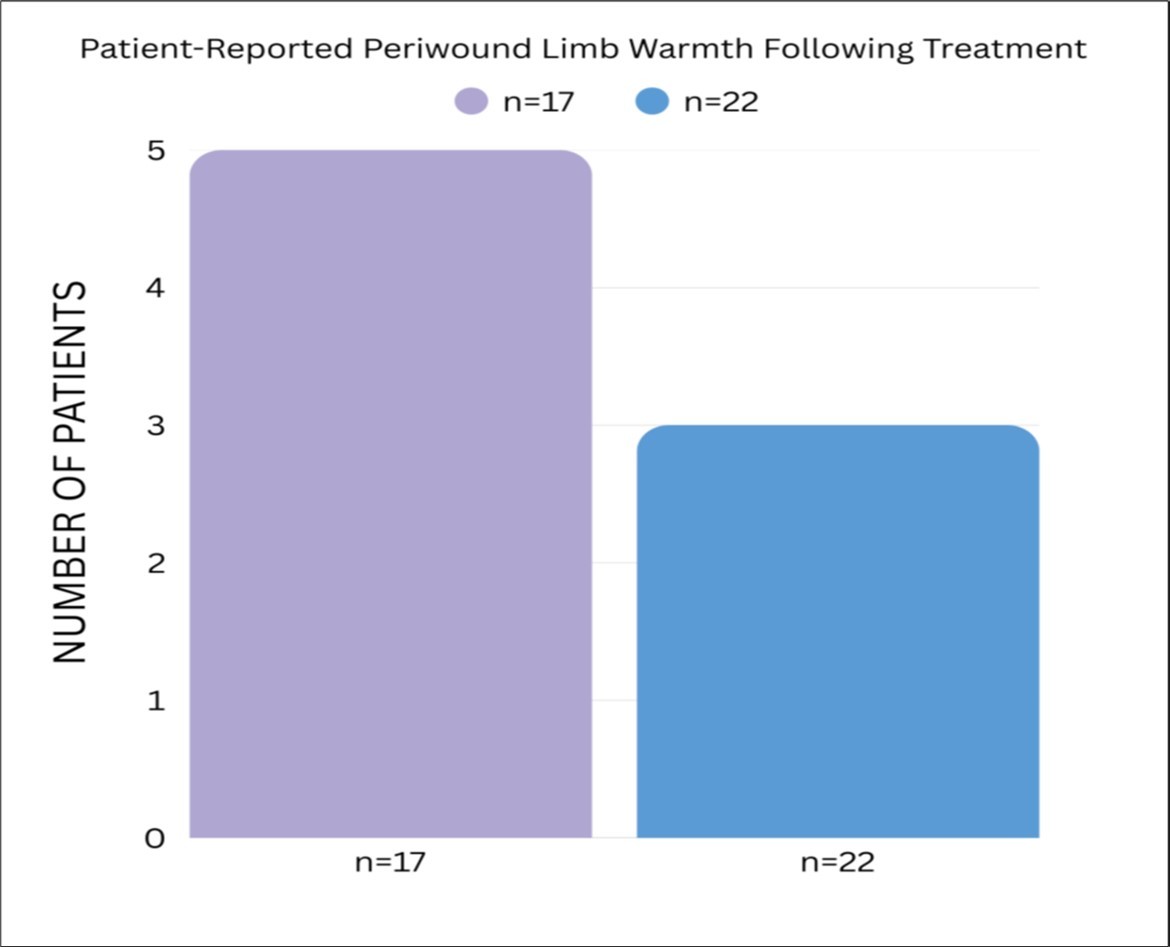
Feasibility Cohort 2 (n = 22)
22 patients with Wagner grade 2–4 chronic diabetic non-healing wounds were treated for one to forty weeks. Eleven patients had been diagnosed for amputation prior to enrollment. Microbe reduction occurred within one to two days, and the wound closure rate was 97 percent. Three patients reported restored warmth, two regained sensation, and thirteen reported increased sensation. All amputations were averted.
Combined Outcomes (n = 39)
Across both feasibility cohorts, the average wound closure rate was 98 percent. All nineteen patients diagnosed for amputation successfully avoided surgical intervention Figure 5, Figure 6.
Figure 5.BROAD CATEGORIES OF BOTH TREATMENT GROUPS (n= 17 and n=22)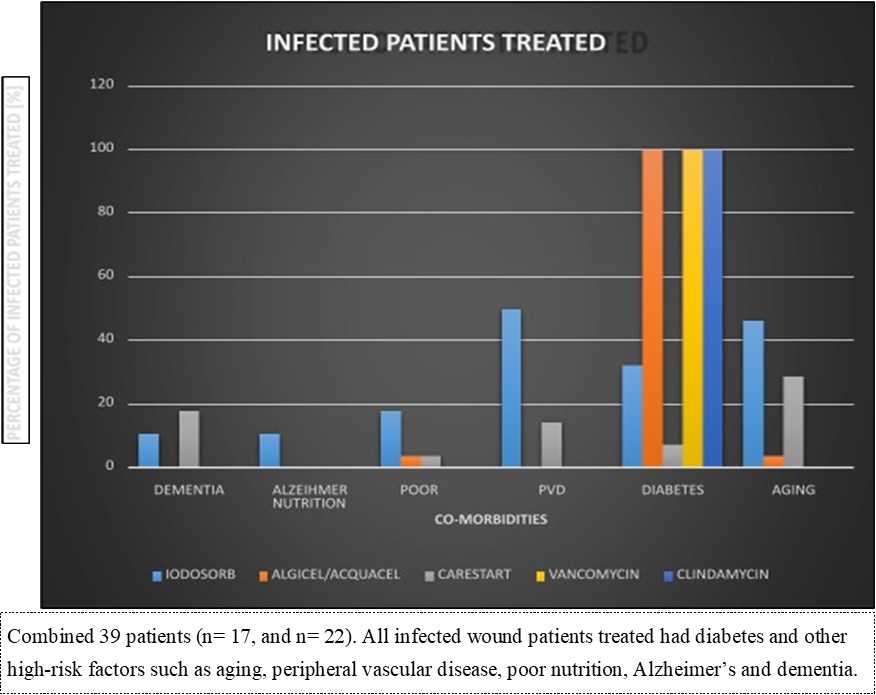
Figure 6.PREVIOUS TREATMENT AND PRODUCTS THAT WERE INEFFECTIVE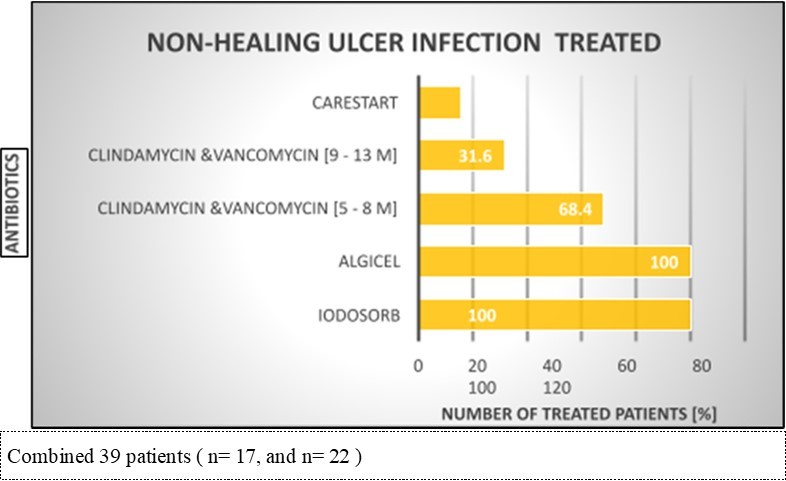
Representative Feasibility Cases
The following cases highlight individual patient outcomes from the Hemastyl studies, demonstrating rapid microbe reduction, nerve regeneration, vascular recovery, and successful wound closure in patients who had previously failed standard care and were diagnosed for amputation. These examples provide real-world clinical context to the aggregate results presented above Figure 7, Figure 8, Figure 9, Figure 10, Figure 11.
Figure 7.By industry’s standard, the wound was non-healing and the leg was recommended for potential future amputation due to frequent recurrent infections. The Hemastyl™ System closed this wound in 50 days and helped the patient avoid an amputation.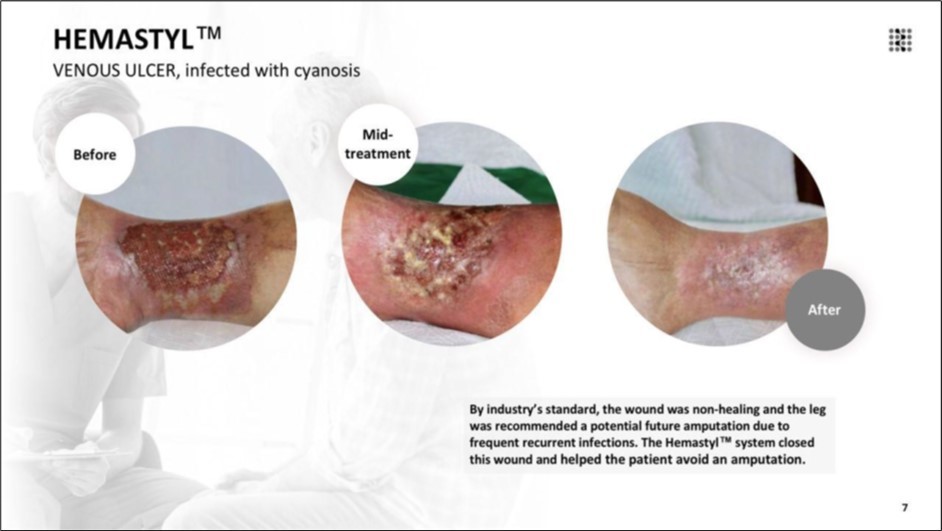
Figure 8.The patient suffered a burn injury and suffered from this chronic wound for 8 years. The Hemastyl™ System eliminated the need for grafting and closed the wound within 6 months.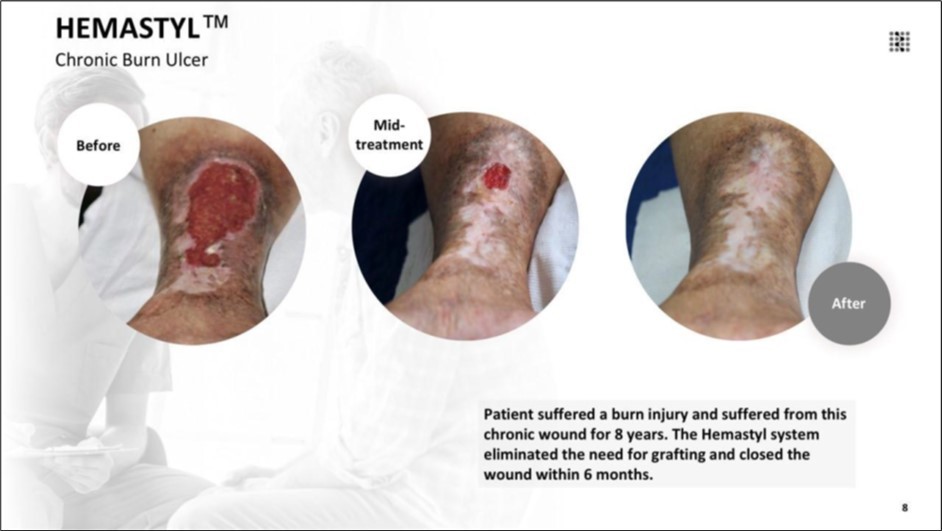
Figure 9.The wound was classified as non-healing according to standard clinical criteria, and the patient was indicated for partial toe amputation. With the Hemastyl™ System, the wound successfully closed in 4 months and the patient avoided amputation.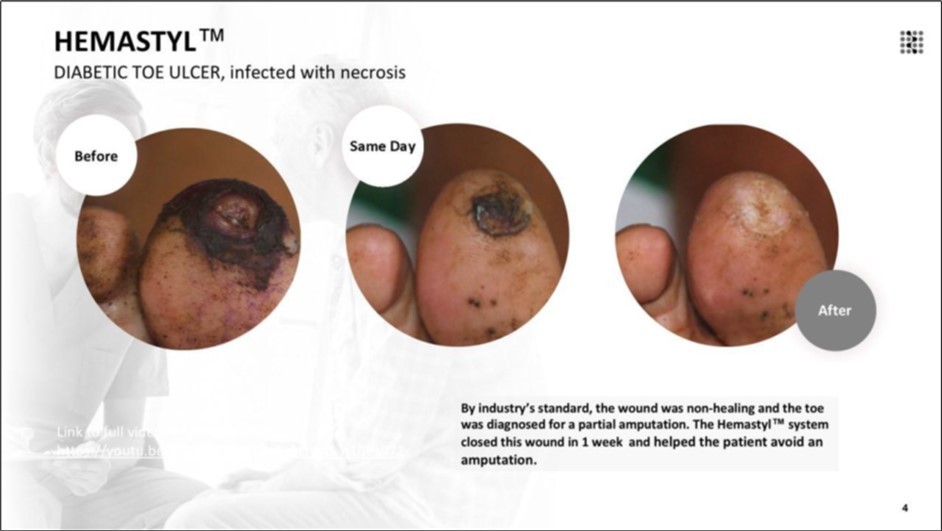
Figure 10.The lesion met clinical thresholds for non-healing and the patient was advised to undergo foot and lower-leg amputation. The Hemastyl™ System restored nerve function and led to full wound closure after 8 months, eliminating the need for amputation.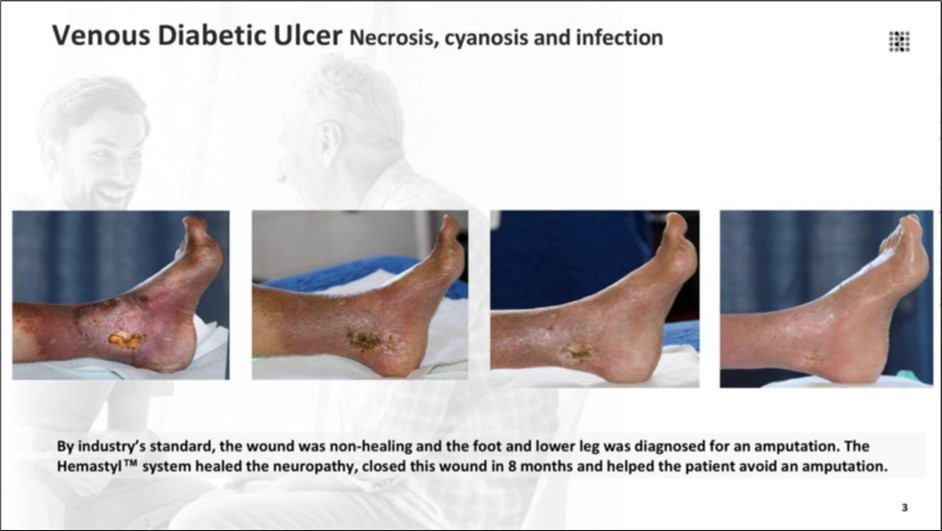
Figure 11.The wound met clinical criteria for a non-healing lesion, and the patient was indicated for lower-leg amputation. The Hemastyl™ System supported sustained periwound recovery and culminated in complete closure at 9 months, negating the need for surgical amputation.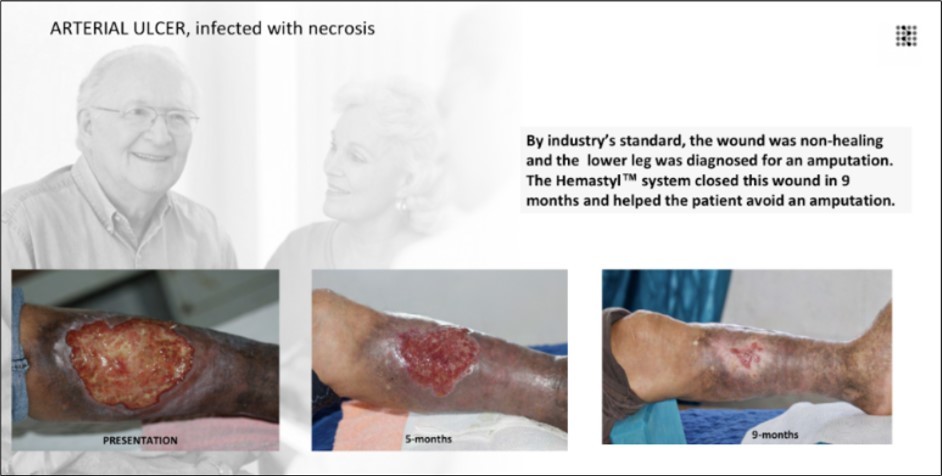
Discussion
Patients enrolled in these feasibility studies represented a high-risk population with long-standing diabetic wounds that had failed standard care for months or years. Many had progressed in disease severity and were facing imminent amputation. Treatment with the Hemastyl™ System resulted in rapid infection reduction, high wound closure rates, and avoidance of amputation in all amputation-diagnosed cases.
Subjective reports of restored warmth and sensation suggest improvement in vascular and neural function, consistent with the system’s intended mechanism of action. These findings support the importance of addressing neuropathy and vascular compromise alongside microbial burden.
Conclusion
The Hemastyl treated ulcers that had failed standard care and were associated with high amputation risk. These feasibility findings suggest that targeting neuropathy, vasculature, and microbes concurrently may offer a promising therapeutic approach for complex chronic wounds.
Ethics Statement
These feasibility studies were conducted as early-stage research investigations in Costa Rica and were not classified as formal clinical trials requiring institutional review board approval.
Informed Consent
Written informed consent was obtained from all participants for participation and for use of de-identified clinical information and photographs for publication.
Funding
External funding for this work was received from The BioRegentech Institute.
References
- 1.D G Armstrong, Boulton A J M, S A Bus. (2017) Diabetic foot ulcers and their recurrence. , New England Journal of Medicine 376(24), 2367-2375.
- 2.A M, Z F Santos, A M Barrera. (2024) A systematic review of diabetic foot infections: Clinical predictors of amputation and outcomes. Frontiers in Clinical Diabetes and Healthcare 5, 1393309.
- 3.McDermott K, Fang M, Boulton A J M, Selvin E, C W Hicks. (2023) Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Clinical Diabetes and Endocrinology. 9(1), 19.
- 4.Zhang Y, Li C, Zhang X. (2023) Global estimates of diabetes-related amputations: Incidence in 2010–2020.
- 5.Netten J J van. (2023) Risk prediction models for diabetic foot ulcer development or amputation: a review of reviews. , Journal of Foot and Ankle Research 16(1), 39.
- 6.J D Raffetto, Mannello F, Jung S, R A Khalil. (2023) Mechanisms of venous ulceration and therapeutic implications. , Journal of Clinical Medicine 12(19), 6153.