Stratified Analysis of Factors Associated With Mortality in Patients With COVID-19 Based on Cancer and Diabetes
Abstract
Background
Cancer and diabetes are risk factors for COVID-19 mortality rates. Remdesivir, dexamethasone, and vaccines are used to improve clinical outcomes. We aimed to evaluate the factors associated with COVID-19 mortality rates.
Methods
This retrospective study enrolled moderate to critical COVID-19 patients. The index day was the day of the COVID-19 diagnosis. Patients were followed up until either death or discharge. A two-way analysis of variance examined the interaction between independent mortality risk factors.
Results
A total of 205 patients were analyzed, and the mortality rate was 29.5% (n=60/205). The cumulative survival rate was significantly lower in patients with a CCI score ≥ 6, cancer, and diabetes. In multivariate analysis, critical illness, cancer, diabetes, chronic liver disease, a CCI score ≥ 6, unvaccinated, and early use of remdesivir/dexamethasone were independent risk factors for mortality. The onset of remdesivir/dexamethasone ≥ 2 days and < 3 doses of vaccinations were higher mortality rate, with its impact being more significant amongst patients with cancer/diabetes, compared to those without cancer/diabetes (p for interaction = 0.046/0.049, 0.060/0.042, and 0.038/0.048 respectively).
Conclusions
COVID-19 vaccination ≥ 3 doses and early administration of remdesivir and dexamethasone can significantly reduce mortality rates, particularly in patients with cancer or diabetes.
Author Contributions
Academic Editor: Ian James Martins, PRINCIPAL RESEARCH FELLOW Edith Cowan University.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2024 Ya-Chun Liao, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors declare no competing interests
Citation:
Introduction
In December 2019, a novel coronavirus, SARS-CoV-2, was first reported in Wuhan, China, causing a substantial impact on the world’s health. The overall global mortality rate of the Coronavirus Disease 2019 (COVID-19) was 1.08%. In Taiwan, a surge of COVID-19 cases was seen from April to June, 2022, with Omicron being the dominant variant reported. Approximately 80,000 new cases were being registered each day. According to a report from the Taiwan Centers for Disease Control (CDC) in June 2020, the mortality rate was around 0.18%.
Several published reports indicated that approximately 81% of people infected with COVID-19 experienced mild disease. Severe disease occupied over 15% of patients, while critical disease took up to 5% 1. According to early data from 2020, the overall mortality rate was 17-24.5% for admitted patients 2, 3. For critically ill patients in an intensive care unit (ICU), the mortality rate was 53-57% 4, 5. Older age, male gender, obesity, immunosuppression, underlying comorbidities of chronic kidney disease (CKD), chronic lung disease (CLD), cardiovascular disease (CVD), neurologic disorders and diabetes were all associated with higher in-hospital mortality rates 2.
The clinical severity varied between the different variants of SARS-CoV-2. According to a case-control study performed in the U.S., in-hospital mortality rates were 7.6% for the alpha variant, 12.2% for delta and 7.1% for omicron 6.The severity of the disease was lower for the omicron variant than before, however, 15% of patients with the omicron variant in this study progressed to invasive mechanical ventilation and 7% eventually to death. For each variant, disease severity was lower in vaccinated patients. Even with the Omicron variant, vaccines remain the most effective tool for protection against COVID-19, particularly amongst patients with underlying comorbidities 7.
As for treatment agents, antiviral therapy and immunomodulators were the most common treatment agents. Remdesivir was used in patients receiving supplemental oxygen or at risk of progression to reduce the clinical recovery time 8, 9, 10. Dexamethasone was also used to improve clinical outcomes and reduce mortality in hospitalized patients under supplemental oxygen 11.
Cancer and diabetes are both clinical risk factors for fatal outcomes associated with COVID-19 12. Among patients with cancer, full vaccination and boosting were associated with improvements in COVID-19 outcomes compared with unvaccinated patients 13. For patients with diabetes, the protective efficiency against mortality from COVID-19 has not yet been discussed. Despite initial evidence showing lower case-fatality rates during the omicron wave than during previous waves 14, the interaction between risk factors (cancer/diabetes) and protective agents (vaccinations/treatment agents) for the outcome of mortality remains unknown. We aimed to investigate the incidence of mortality rates amongst patients experiencing moderate to critical illness of COVID-19, and the factors associated with mortality based on cancer and diabetes diagnoses.
Materials and Methods
Study design and ethics standards
This retrospective, observational cohort study enrolled moderate to critical illness COVID-19 patients admitted to isolation rooms from April 1 to June 30, 2022, at Taichung Veterans General Hospital. Patients aged <18 years and pregnant were excluded. The study was approved by the Taichung Veterans General Hospital Human Studies Committee.
Patient definition and data collection
COVID-19 patients were defined by a positive SARS-CoV-2 infection test result, confirmed by real time reverse transcriptase–polymerase chain reaction (RT-PCR) assay from nasal and pharyngeal swabs. According to COVID-19 severity definitions established by the Taiwan CDC and American College of Emergency Physicians (ACEP), moderate illness was defined as having signs and symptoms of lower respiratory disease or abnormal imaging and a SpO2 ≥ 94% at room temperature at sea level. Severe illness was defined as a SpO2 < 94% at room temperature at sea level, a PaO2/FiO2 < 300 mmHg, a respiratory frequency > 30 breaths/minute or having lung infiltrates > 50%. Critical illness was defined as respiratory failure, septic shock, and/or multiple organ dysfunction. The index day (day 0) was the day when a SARS-CoV-2 RT-PCR test confirmed positive, with patients followed until death in hospital or hospital discharge, whichever occurred first.
Medical chart reviews were conducted by infectious disease doctors using a standard case report form. We recorded each patient’s age, gender, body mass index (BMI) and preexisting comorbidities, including cancer, CVD, diabetes, CKD, CLD and chronic liver disease. Obesity was defined as a calculated BMI ≥ 30 kg/m2. The Charlson comorbidity index (CCI), used to estimate the risk of death from comorbid diseases, was used for calculations.15 We also recorded each patient’s history of COVID-19 vaccinations. In Taiwan, four types of vaccines, Moderna, AstraZeneca (AZ), Pfizer-Biontech (BNT) and the MVC-COV1901 COVID-19 Vaccine, were available during the study period. Groups of respiratory support were stratified from non-oxygen use/nasal cannula, to simple mask/non-rebreathing mask/high-flow nasal cannula (HFNC), and bilevel positive airway pressure (BiPAP)/mechanical ventilators. Time to remdesivir or dexamethasone was identified as the onset of COVID-19 symptoms to medication administration.
Statistical analysis
The demographic data regarding the survivors and non-survivors were analyzed. Descriptive statistics for patient and hospital characteristics were calculated using either mean (standard deviation (SD)), median (range or interquartile range (IQR)), or frequency count (percentage). Continuous variables were analyzed by the Wilcoxon rank-sum test and categorical variables by the Chi-square test or Fisher’s exact test. All tests were two-sided, and a p value of <0.05 was considered to be statistically significant. The Kaplan-Meier method was used to determine the cumulative survival rate, category by CCI score, cancer and diabetes. Logistic regression analysis was used to determine independent predictors of hospital mortality. Model 1 was adjusted for age, gender, severity of COVID-19, cancer, CVD, diabetes, CKD, CLD, chronic liver disease, obesity, history of vaccination, remdesivir use, dexamethasone use and the cycle threshold (Ct) value at day 0. Model 2 was adjusted for age, gender, severity of COVID-19, obesity, CCI ≥ 6, history of vaccination, remdesivir use, dexamethasone use and Ct value at day 0. Variables differing between survivors and non-survivors with a p value < 0.5, according to the Chi-square test or Fisher’s exact test, or the Wilcoxon rank-sum test, were entered into the multivariable regression model. Results were presented as odds ratios (ORs) with 95% confidence intervals (CI). Two-way analysis of variance (ANOVA) was used to examine the interaction between the independent risk factors for hospital mortality, including the onset of remdesivir and dexamethasone use time, history of vaccinations, cancer and diabetes. Statistical analysis were performed using SPSS software (Version 22.0).
Results
Patient enrollment
A total of 838 patients were assessed for eligibility, with 196 children (23.39%) and 21 pregnant women (2.5%) being excluded. 416 patients were excluded due to mild COVID-19 illness. Ultimately, 205 patients experiencing moderate to critical COVID-19 illness were enrolled in the final analysis (Figure 1). Amongst them, 60 patients (29.27%) expired and 145 (70.73%) survived, with the mortality rate within the moderate to critical illness COVID-19 patients being 29.5%.
Clinical characteristics in the moderate to critical COVID-19 patients
Clinical characteristics within the moderate to critical COVID-19 patients are shown in Table 1. The median age was 73.0 years for survivors and 78.5 years for non-survivors. Non-survivors were more likely to be experiencing critical illness and also have a higher proportion of cancer, diabetes and CVD. The median CCI score was significantly higher in non-survivors (7.5) compared with survivors (5.0). Only 21.7% of non-survivors received ≥ 3 doses of the COVID-19 vaccinations. With respiratory support, 40.0% of non-survivors required Bi-PAP or mechanical ventilator respiratory support. For COVID-19 treatment, oral antiviral agents included nirmatrelvir/ritonavir (Paxlovid) and Molnupiravir, with 16 patients (16/205, 7.8%) receiving Paxlovid and 31 patients (31/205, 15.12%) receiving Molnupiravir in the survivor group. In the non-survivor group, only 14 patients (14/205, 6.82%) received Molnupiravir, with no patients receiving Paxlovid. The onset of remdesivir and dexamethasone use time was more likely to be more than 2 days for non-survivors. An empirical antibiotic was used in 198 patients (198/205, 96.59%), 97.24% of survivors, and 95% of non-survivors. The overall length of hospital stay was 14.91 (7-20) days, 15.31(10-20) days for survivors, and 14.23 (1-58) days for non-survivors.
Table 1. Clinical characteristics of COVID-19 patients.| Characteristic | All patients(n=205)N(%) | Survivors(n=145)N(%) | Non-survivors(n=60)N(%) | P value |
| Demographics | ||||
| AgeMean(±standard deviation)Median(25th,75th percentiles) | 72.66(±15.86)74.00(63,85) | 72.03(±15.66)73.00(62,85) | 74.18(±16.37)78.50(63,87) | 0.356 |
| Gender(male) | 135(65.9%) | 97(66.9%) | 38(63.3%) | 0.666 |
| Severity of COVID-19 | ||||
| Moderate | 64(31.2%) | 50(34.5%) | 14(23.3%) | 0.254 |
| Severe | 107(52.2%) | 83(57.2%) | 24(40.0%) | 0.197 |
| Critical | 34(16.6%) | 12(8.3%) | 22(36.7%) | <0.001* |
| Comorbidities | ||||
| Cancer | 62(30.2%) | 36(24.8%) | 26(43.3%) | <0.001* |
| Cardiovascular disease | 79(38.5%) | 52(35.9%) | 27(45.0%) | 0.057 |
| Diabetes | 78(38.0%) | 49(33.8%) | 29(48.3%) | 0.007* |
| Chronic kidney disease | 39(19.0%) | 28(19.3%) | 11(18.3%) | 0.552 |
| Chronic lung disease | 28(13.7%) | 22(15.2%) | 6(10.0%) | 0.211 |
| Chronic liver disease | 14(6.8%) | 6(4.1%) | 8(13.3%) | 0.038* |
| Obesity | 10(5.7%) | 7(5.8%) | 3(5.5%) | 0.931 |
| Charlson comorbidity indexMean(±standard deviation)Median(25th,75th percentiles) | 6.17(±3.23)6.00(4,8) | 5.41(±2.96)5.00(3,7) | 8.00(±3.16)7.50(6,10) | <0.001* |
| History of vaccination | 0.020* | |||
| 0 dose | 105(51.7%) | 71(49.7%) | 34(56.7%) | |
| 1-2 doses | 40(19.7%) | 27(18.9%) | 13(21.7%) | |
| ≥3rd dose | 58(28.6%) | 45(31.5%) | 13(21.7%) | |
| Ct value at day 0 | 18.67(14-21) | 18.62(15-21) | 18.79(13-23) | 0.942 |
| Group of respiratory support | <0.001* | |||
| Non-oxygen use/Nasal cannula | 86(42.0%) | 77(53.1%) | 9(15.0%) | |
| Simple mask/Non-rebreathing/HFNC | 56(27.3%) | 29(20.0%) | 27(45.0%) | |
| Bi-PAP/Ventilator | 63(30.7%) | 39(26.9%) | 24(40.0%) | |
| COVID-19treatment | ||||
| Remdesivir use group | <0.001* | |||
| No remdesivir use | 30(14.6%) | 17(11.7%) | 13(21.7%) | |
| Onset of remdesivir use time<2 days | 94(45.9%) | 83(57.3%) | 11(18.3%) | |
| Onset of remdesivir use time≥2 days | 81(39.5%) | 45(31.0%) | 36(60.0%) | |
| Dexamethasone use group | <0.001* | |||
| No dexamethasone use | 72(35.1%) | 47(32.4%) | 25(41.7%) | |
| Onset of dexamethasone use time<2days | 76(37.1%) | 65(44.8%) | 11(18.3%) | |
| Onset of dexamethasone use time≥2days | 57(27.8%) | 33(22.8%) | 24(40.0%) | |
| IL-6 inhibitor use | 3(1.5%) | 2(1.4%) | 1(1.7%) | 0.939 |
| Oral Paxlovid or Molnupiravir use | 61(30.0%) | 47(32.6%) | 14(23.7%) | 0.179 |
Cumulative survival rate
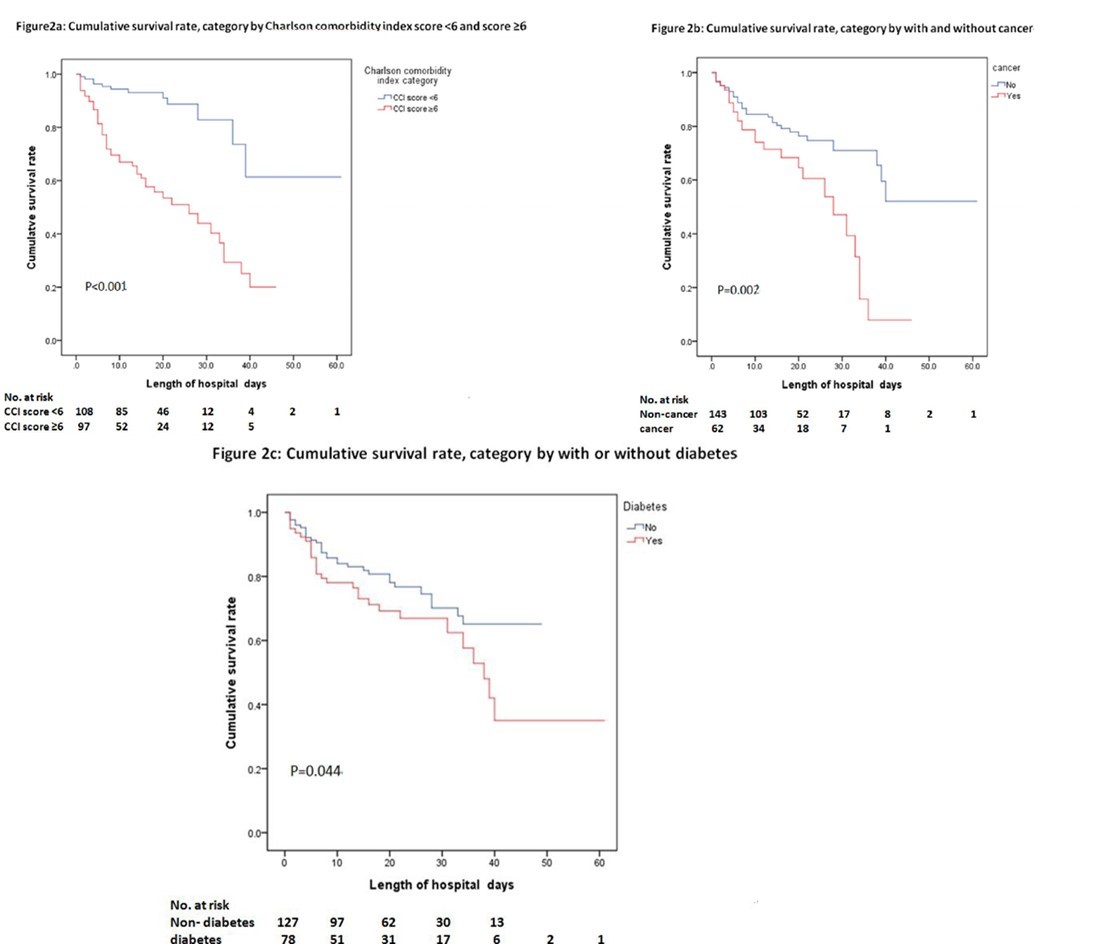
The cumulative survival rates during admission are shown in Figure 2a, Figure 2b and Figure 2c. The cumulative survival rate amongst patients with a CCI score ≥ 6 was significantly lower than those with a CCI score < 6 (P < 0.001) (Figure 2a). The cumulative survival rate amongst patients with cancer was significantly lower than those without cancer (P = 0.002) (Figure 2b). The cumulative survival rate amongst patients with diabetes was significantly lower than those without diabetes (P = 0.044) (Figure 2c).
Multivariable analysis
Multivariable analysis for COVID-19 mortality risk factors is shown in Table 2. Critical illness of COVID-19 (Model 1: OR=6.55; 95% CI, 2.61–16.43; Model 2: OR=6.13; 95% CI, 2.09–17.97), underlying comorbidities of cancer (OR=2.35; 95% CI, 1.47–3.76), diabetes (OR=2.32; 95% CI, 1.23–4.37), chronic liver disease (OR=3.22; 95% CI, 1.01–10.29), and a CCI score ≥ 6 (OR=7.77; 95% CI, 2.70–22.35) were significantly associated with a higher risk of mortality. Patients who never received any vaccination (Model 1: OR=3.22; 95% CI, 1.07–9.66; Model 2: OR=3.57; 95% CI, 1.12–11.36), had an onset of remdesivir use time ≥ 2 days (Model 1: OR=6.04; 95% CI, 2.81–12.99; Model 2: OR=7.89; 95% CI, 1.34–16.40), and had an onset of dexamethasone use time ≥ 2 days (Model 1: OR=4.30; 95% CI, 1.88–9.83; Model 2: OR=4.47; 95% CI, 1.34–22.39) were all independent risk factors for mortality in both Models 1 and 2.
Table 2. Logistic regression analysis of the risk factors associated with survivor and non-survivor outcomes in COVID-19 patients.| Variable | Univariable analysis | Multivariable analysis(Model 1) | Multivariable analysis (Model 2) | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Demographics | ||||||
| Age (odds ratio reported per one-year increase), years | 1.01 (0.99-1.03) | 0.376 | 1.02 (0.99-1.04) | 0.218 | 0.99 (0.97-1.02) | 0.634 |
| Gender (male) | 1.17 (0.62-2.19) | 0.625 | 1.15 (0.61-2.18) | 0.665 | 1.61 (0.71-3.68) | 0.254 |
| Severity of COVID-19 | ||||||
| Moderate | 1.00 | - | 1.00 | - | 1.00 | - |
| Severe | 4.47 (0.38-51.96) | 0.232 | 1.03 (0.49-2.18) | 0.933 | 1.13 (0.47-2.73) | 0.783 |
| Critical | 9.32 (4.19-20.71) | <0.001* | 6.55 (2.61-16.43) | <0.001* | 6.13 (2.09-17.97) | <0.001* |
| Comorbidities | ||||||
| Cancer | 3.96 (1.65-9.46) | 0.002* | 2.35 (1.47-3.76) | <0.001* | ||
| Cardiovascular disease | 1.79 (0.89-3.57) | 0.100 | 1.46 (0.79-2.70) | 0.222 | ||
| Diabetes | 2.64 (1.30-5.35) | 0.007* | 2.32 (1.23-4.37) | 0.010* | ||
| Chronic kidney disease | 0.76 (0.33-1.77) | 0.529 | 0.94 (0.43-2.03) | 0.871 | ||
| Chronic lung disease | 0.52 (0.19-1.46) | 0.216 | 0.62 (0.24-1.62) | 0.330 | ||
| Chronic liver disease | 3.56 (1.18-10.77) | 0.024* | 3.22 (1.01-10.29) | 0.048* | ||
| Obesity | 1.13 (0.24-5.36) | 0.880 | 0.99 (0.23-4.38) | 0.993 | 0.94 (0.23-3.78) | 0.930 |
| Charlson comorbidity index≥6 | 7.84 (3.81-16.10) | <0.001* | 7.77 (2.70-22.35) | <0.001* | ||
| History of vaccination | ||||||
| ≥3rd dose | 1.00 | - | 1.00 | - | 1.00 | - |
| 1-2 doses | 3.91 (0.86-17.87) | 0.079 | 1.92 (0.85-4.37) | 0.119 | 1.01 (0.35-2.87) | 0.192 |
| 0 dose | 5.46 (1.57-18.98) | 0.008* | 3.22 (1.07-9.66) | 0.037* | 3.57 (1.12-11.36) | 0.031* |
| COVID-19 treatment | ||||||
| Remdesivir use | ||||||
| Onset of remdesivir use time<2 days | 1.00 | - | 1.00 | - | 1.00 | - |
| No remdesivir use | 7.85 (3.63-16.96) | <0.001* | 5.77 (2.22-15.03) | <0.001* | 4.58 (1.03-20.40) | 0.046* |
| Onset of remdesivir use time≥2 days | 9.31 (3.26-26.55) | <0.001* | 6.04 (2.81-12.99) | <0.001* | 7.89 (1.34-16.40) | 0.002* |
| Dexamethasone use | ||||||
| Onset of dexamethasone use time<2days | 1.00 | - | 1.00 | - | 1.00 | - |
| No dexamethasone use | 4.73 (2.02-11.04) | <0.001* | 3.14 (1.41-7.01) | 0.005* | 3.58 (1.20-10.66) | 0.022* |
| Onset of dexamethasone use time≥2days | 5.63 (2.42-13.07) | <0.001* | 4.30 (1.88-9.83) | 0.001* | 4.47 (1.34-22.39) | 0.018* |
| Ct value at day 0 | 1.01 (0.95-1.06) | 0.846 | - | - | - | - |
Interaction between the independent risk factors and mortality
Table 3 shows the association between cancer, diabetes and the onset of remdesivir use time for mortality rates. An onset of remdesivir use time (≥ 2 days) was associated with an increased mortality rate, particularly amongst patients with cancer/diabetes, compared to those without cancer/diabetes (p for interaction = 0.046/0.049). Table 4 shows the association between cancer, diabetes and the onset of dexamethasone use time for mortality rates. An onset of dexamethasone use time (≥ 2 days) was associated with a higher mortality rate in patients with cancer. In addition, the impact was significant amongst patients with diabetes, compared to those without diabetes (p for interaction = 0.042). Table 5 shows the association between cancer, diabetes and COVID-19 vaccines. Compared to patients who received ≥ 3 doses of COVID-19 vaccinations, those who received < 3 doses were associated with a higher mortality rate, particularly amongst patients with cancer/diabetes, compared to those without cancer/diabetes (p for interaction = 0.038/0.048).
Table 3. Association between cancer, diabetes and onset of remdesivir use time in survivors and non-survivors. (n=205)| Group | Onset of remdesivir use time | aOR (95% CI) | P value | P for interaction |
| Cancer | aOR# | 0.046* | ||
| No | <2 days use | 1.00 | - | |
| No use | 0.56 (0.23-1.35) | 0.196 | ||
| ≥2 days use | 0.85 (0.47-1.55) | 0.595 | ||
| Yes | <2 days use | 1.00 | - | |
| Non use | 1.75 (1.07-2.86) | 0.026* | ||
| ≥2 days use | 2.93 (1.65-5.20) | <0.001* | ||
| Diabetes | aOR※ | 0.042* | ||
| No | <2 days use | 1.00 | - | |
| No use | 0.69 (0.26-1.82) | 0.432 | ||
| ≥2 days use | 0.82 (0.41-1.65) | 0.576 | ||
| Yes | < 2 days use | 1.00 | - | |
| No use | 2.84 (1.14-7.07) | 0.025* | ||
| ≥2 days use | 3.72 (1.46-9.44) | 0.006* |
| Group | Onset of dexamethasone use time | aOR (95% CI) | P value | P for interaction |
| Cancer | aOR# | 0.06 | ||
| No | <2 days use | 1 | - | |
| No use | 0.44 (0.12-1.66) | 0.225 | ||
| ≥2 days use | 0.58 (0.20-1.71) | 0.323 | ||
| Yes | < 2 days use | 1 | - | |
| No use | 2.49 (1.12-5.54) | 0.046* | ||
| ≥2 days use | 3.13 (1.40-7.03) | 0.006* | ||
| Diabetes | aOR※ | 0.042* | ||
| No | <2 days use | 1 | - | |
| No use | 0.69 (0.26-1.82) | 0.432 | ||
| ≥2 days use | 0.82 (0.41-1.65) | 0.576 | ||
| Yes | < 2 days use | 1 | - | |
| No use | 2.84 (1.14-7.07) | 0.025* | ||
| ≥2 days use | 3.72 (1.46-9.44) | 0.006* |
| Group | History of vaccination | aOR (95% CI) | P value | P for interaction |
| Cancer | aOR# | 0.038* | ||
| No | ≥3rd dose | 1.00 | - | |
| <3rd dose | 1.67 (1.12-2.52) | 0.015* | ||
| Yes | ≥3rd dose | 1.00 | - | |
| <3rd dose | 4.24 (1.74-10.36) | 0.002* | ||
| Diabetes | aOR※ | 0.048* | ||
| No | ≥3rd dose | 1.00 | - | |
| <3rd dose | 1.62 (1.00-2.62) | 0.029* | ||
| Yes | ≥3rd dose | 1.00 | - | |
| <3rd dose | 2.74 (1.31-5.76) | 0.008* |
Discussion
In this observational retrospective study, we found even during the omicron wave, in-hospital mortality was still high (29.5%) among moderate to critical illness COVID-19 patients. Cancer, diabetes, chronic liver disease and a CCI score ≥ 6 were all independent risk factors for mortality. Being COVID-19 vaccinated with ≥ 3 doses, the onset of both remdesivir and dexamethasone for < 2 days were protective factors for mortality. The protective effect of ≥ 3 doses of the vaccine, along with early administration of remdesivir and dexamethasone can greatly reduce mortality rates, particularly in patients with cancer or diabetes.
The overall in hospital mortality rate of COVID-19 ranges from 17 to 24.5% 2, 3. Our study showed a higher in-hospital mortality rate (29.5%) than previous studies. Additionally, 30.7% of patients in this study saw progression to BiPAP or invasive mechanical ventilator, which may be due to the relatively old age (median age 74 years) of our patients and a higher percentage of underlying comorbidities, particularly cancer and diabetes. The results of our study also emphasize the relatively high mortality rate in those with multiple comorbidities despite the omicron wave.
As for the treatment of COVID-19 patients, the WHO Solidarity trial determined that remdesivir shortens the time to clinical recovery 16. Similarly, the PINETREE trial and CATCO study also demonstrated the clinical efficacy of remdesivir in hospitalized patients. The further investigation surrounding the timing of remdesivir has suggested that early-remdesivir use could prevent a progression to severe COVID-19 pneumonia 10, 17. One prospective, observational study indicated that early-remdesivir use (≤ 5 days from onset of symptoms) was a protective factor, and may reduce COVID-19 progression 18. Another retrospective observational study also found patients receiving remdesivir ≤ 3 days from first testing positive for SARS-CoV-2 had 1.74 times a reduction in mortality rate compared to those receiving remdesivir after > 3 days 19. Our study also demonstrated the benefits of early remdesivir administration. An onset of remdesivir use time ≥ 2 days was an independent risk factor for in-hospital mortality rates, particularly amongst patients with cancer and diabetes.
Host immune response is thought to play a central role in the pathophysiological effects of organ failure in severe COVID-19 patients. Corticosteroids have been used as immunomodulators to reduce COVID-19 related systemic inflammatory response. The RECOVERY trial demonstrated the use of dexamethasone resulted in a lower 28-day mortality rate amongst those receiving oxygen supplement 11. The benefits of early administration of dexamethasone remain controversial. One retrospective study showed that severe COVID-19 patients receiving corticosteroids within 10 days from diagnosis had a favorable outcome in the length of their hospital stay 20. Another observational study found that early administration of dexamethasone (within 24 hours of hypoxemia) was associated with a lower rate of HFNC and mechanical ventilator appliance 21, however, another observational cohort study did not show any mortality benefits 22.In our study, the onset of dexamethasone use time ≥ 2 days was also an independent risk factor for in-hospital mortality rates, particularly among patients with cancer or diabetes. Our study supported the early timing of dexamethasone use. However, the benefit was more significant for critical condition patients. Early consideration of corticosteroid use should be recommended, particularly in those patients with high-risk comorbidities, to limit the rapid progression of COVID-19.
Vaccines remain the most valuable instrument in the protection against COVID-19. In a previous study, participants being 60 years old or older showed that the rates of confirmed COVID-19 and severe illness were substantially lower amongst those receiving a booster (third) dose of the BNT162b2 vaccine 23. Even during the Omicron-dominant epidemic period, the risk of mortality decreased with each additional vaccine and protection against death among those aged > 50 years was more significant 24. Among patients with cancer, full vaccination and boosting were associated with an improvement in COVID-19 related mortality and morbidity rates compared with unvaccinated patients 13. Similarly, our study highly emphasizes the effectiveness of a booster (third) dose of a COVID-19 vaccine, as it reduced mortality rate during the omicron phase, particularly amongst patients with diabetes and cancer.
There were some limitations in our study. First, this study was a single medical center with a small patient number, selection bias existed. Secondly, our database did not contain certain patient personal information, such as smoking and alcohol drinking habits, which are associated with increased severity of disease and death for hospitalized COVID-19 patients 25, 26, 27. We cannot analyze patient histories regarding smoking and alcohol consumption due to poor data quality. Finally, we only followed up with patients until discharge, so there were no long-term survival outcome results.
In summary, patients being COVID-19 vaccinated with ≥ 3 doses, and undergoing an early onset of remdesivir or dexamethasone of < 2 days can experience a greatly reduced mortality rate, particularly in patients with cancer or diabetes.
Declarations
Ethics approval and consent to participate
Since our study was retrospective and mainly used data from existing medical records, it was anonymized when analyzed, protecting the participants' privacy and not imposing additional risks or burdens on them. Therefore, the ethics committee (Taichung Veterans General Hospital Human Studies Committee) waived the requirement for informed consent. (approval No. CF22345B) on Sep 29, 2022.
No potentially identifable human images or data is presented in this study.
Consent for publication
Not applicable
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
None received.
Acknowledgements
The authors would like to thank all the patients for participating in this study and the staff of the COVID-19 Care Unit in Taichung Veterans General Hospital.
References
- 1.Wu Z, McGoogan J M. (2020) Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention.JAMA. 323, 1239-1242.
- 2.Kim L, Garg S, O'Halloran A. (2021) Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET).Clin Infect Dis,72:e206-e214.
- 3.Richardson S, Hirsch J S, Narasimhan M. (2020) . Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the , New York City, Area.JAMA 323, 2052-2059.
- 4.Grasselli G, Greco M, Zanella A. (2020) Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy. , Italy.JAMA Intern Med 180, 1345-1355.
- 5.Estenssoro E, Loudet C I, Rios F G. (2021) Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): A prospective, multicentre cohort study.Lancet Respir Med. 9, 989-998.
- 6.Lauring A S, Tenforde M W, Chappell J D. (2022) Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study.BMJ. 376-069761.
- 7.VKCB Yan, EYFP Wan, Ye X M. (2022) . Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: A case-control study.Emerg Microbes Infect 1-48.
- 8.Beigel J H, Tomashek K M, Dodd L E. (2020) Remdesivir for the Treatment of Covid-19 -. , Final Report.N Engl J Med 383, 1813-1826.
- 9.Ader F, Bouscambert-Duchamp M, Hites M. (2022) Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial.Lancet Infect Dis. 22, 209-221.
- 10.Ali K, Azher T, Baqi M. (2022) Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial.CMAJ.
- 11.Group R C, Horby P, Lim W S. (2021) . Dexamethasone in Hospitalized Patients with Covid-19.N Engl J Med 384, 693-704.
- 12.Dessie Z G, Zewotir T. (2021) Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients.BMC Infect Dis. 21, 855.
- 13.Pinato D J, Aguilar-Company J, Ferrante D. (2022) Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: Results from the retrospective, multicentre, OnCovid registry study.Lancet. , Oncol 23, 865-875.
- 14.Maslo C, Toubkin M. (2022) Characteristics and Outcomes of Hospitalized Patients. in South Africa During the COVID-19 Omicron Wave-Reply.JAMA 327, 2148.
- 15.Charlson M E, Pompei P, Ales K L. (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation.J Chronic Dis. 40, 373-83.
- 16.WHOST Consortium. (2022) Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses.Lancet. 399, 1941-1953.
- 17.Gottlieb R L, Vaca C E, Paredes R. (2022) Early Remdesivir to Prevent Progression to Severe Covid-19. , in Outpatients.N Engl J Med 386, 305-315.
- 18.Falcone M, Tiseo G, Barbieri C. (2022) . Early Use of Remdesivir and Risk of Disease Progression in Hospitalized Patients With Mild to Moderate COVID-19.Clinical Therapeutics 44, 364-373.
- 19.Paranjape N, Husain M, Priestley J. (2021) Early Use of Remdesivir in Patients Hospitalized With COVID-19 Improves Clinical Outcomes: A Retrospective Observational Study.Infect Dis Clin Pract (Baltim Md).
- 20.Hyun J H, Kim M H, Sohn Y. (2021) Effects of early corticosteroid use in patients with severe coronavirus disease 2019.BMC Infect Dis. 21, 506.
- 21.Lee H W, Park J, Lee J K. (2021) . The Effect of the Timing of Dexamethasone Administration in Patients with COVID-19 Pneumonia.Tuberc Respir Dis (Seoul) 84, 217-225.
- 22.Crothers K, DeFaccio R, Tate J. (2022) Dexamethasone in hospitalised COVID-19 patients not on intensive respiratory support.Eur Respir. , J 60.
- 23.Bar-On Y M, Goldberg Y, Mandel M. (2021) . Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel.N Engl J Med 385, 1393-1400.
- 24.VKC Yan, EYF Wan, Ye X. (2022) Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: A case-control study.Emerg. , Microbes Infect 11, 2304-2314.
- 25.Zhao Q, Meng M, Kumar R. (2020) The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis.J. , Med Virol 92, 1915-1921.