Abstract
The cactus Opuntia ficus indica (L.) Mill. (Cactaceae) is widely used in Tunisian medicine for the treatment of various illnesses. The purpose of the present study was to investigate the antioxidant activities of cactus cladode extract (CCE) and to assess the protective effects of Opuntia ficus indica against osteoporosis induced by cadmium chloride in male Wistar rats. Adult male rats were divided into 4 groups of 9 each: a control group, a group injected with cadmium (3.5mg/kg) for 10 weeks, a group orally given a O. ficus indica cladodes aqueous extract (CCE) (100 mg /kg/day) for 10 weeks then treated with cadmium, and a group receiving only (CCE) for 10 weeks. Bone toxicity was estimated by examining femoral length and weight, calcium, phosphorus, alkaline phosphatase (ALP) and total tartrate-resistant acid phosphatase (ACP) levels in serum. Also, bone levels of calcium, phosphorus and vitamin C and bone mineral density (BMD) of femur diaphysis were measured. Alterations of these bone biomarkers and decreased BMD confirmed cadmium-induced bone toxicity. However, when cadmium was administered in rats given CCE, all the biological parameters underwent much less alteration. Administration of CCE was found to be beneficial by attenuating cadmium-induced femur damage. The protective effect of the plant is mainly attributed to its phenolic compounds that orchestrate antioxidant properties, as highlighted by HPLC-based analysis.
Author Contributions
Academic Editor: Fatma mohammed Mady, Department of Pharmaceutics, Minia University, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Jihen Taleb, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
No author has any associations that may represent a potential conflict of interest.
Citation:
Introduction
Cadmium (Cd) is one of the important environmental polluant. Food and tobacco are the main source of Cd exposure in general population 1. Cd can be found in certain drinks, meat, grains and in cigarette. Therefore, important origins of Cd consumption are via food or cigarette 2. Although, extended cadmium exposure can induce various damages, including renal dysfunction, liver injuries, and cardiovascular diseases 3, 4. Due to its long biological half-life in the body (more than 10 years), low level of Cd exposure also could cause bone failure which may increase the risk of osteoporosis and bone fractures 5, 6.
Long-term exposure to Cd results in nephron disturbances, raised excretion of calcium, generalized osteomalacia and osteoporosis due to competitive displacement of calcium ions from bone tissue and degeneration of the bone structure, which induces frequent bone fractures and cracking 7.
In the recent years, alternative therapeutic approaches have become very popular, it has been a growing attention, in natural antioxidants due to their virtues (such as liver, kidney, or immune system problems) when used for medicinal target 8, 9, 10.
In addition, numerous studies have shown that bioactive compounds, such as green tea catechins, daidzein and quercetin are also valuable in protecting against Cd toxicity 11. However, only limited studies focused on the treatments of Cd-induced osteoporosis. Recently, virgin olive oil and ginger may be beneficial in protecting against Cd induced bone damage 12, 13.
O.ficus indica (L.) Mill. (Cactaceae) is a cactus species widely distributed in Latin America, South Africa and the Mediterranean area. Young cladodes have been used as vegetables in deferent forms and they are applied to be health-promoting food since they contain a great number of bioactive nutrients. Particularly, the high calcium fibers and phenolics contents are worth to be described in cactus cladodes 14. Therefore, cladodes powder was proposed as ingredient in milk-based drinks and breakfast cereals. Furthermore, it may be applied up to 20% as a thickening agent in vegetable soups 15. Recently, cladodes enrichment was found to be promising in terms of fat retention and oxidative damage reduction, resulting in more stable product 16.
Cactus cladodes were proved successful in protecting several organs against diseases in diverse experimental models 17. O. ficus indica yields great values of major nutrients such as minerals, carotenoids, fatty acids, and essential oil and vitamins 18, 19. This desert plant can be used as an anti-inflammatory, hypoglycemic, antiviral and may protect against several chronic diseases including cancer and neurological diseases 17, 20. Furthermore, previous studies confirmed its hepatoprotective, immunoprotective and antioxidant capacities against heavy metals toxicity 21, 22. However, thus far therapeutic effects of O. ficus indica extract against Cd-induced skeletal damages have not been investigated. This study evaluates the preventive effects of O. ficus indica against bone damage induced by cadmium administration in male rats. Finally, phytoscreening of the cactus extract was carried out to identify its bioactive compounds.
Materials and Methods
Chemicals
Cadmium chloride (CdCl2) was obtained from Merck (Darmstadt, Germany) and dissolved in sodium chloride (saline, 0.9% NaCl). Methanol 80%, acetone, ascorbic acid, potassium ferricyanide solution, acetic acid, phosphate buffer, trichloroacetic acid, solution thiobarbituric acid (TBA), ferric chloride, ferrozine, nitric acid, butylated hydroxytoluene (BHT), dinitrophenyl hydrazine, CuSO4 Tris-HCl buffer, ethylene diamine tetraacetic acid (EDTA) and aluminum chloride (AlCl3) were purchased from Sigma (Sigma, Aldrich). Gallic acid, rutin, and catechin were purchased from Merck (Darmstadt, Germany). Commercial diagnostic kits were purchased from Biomaghreb (Tunisia). All the other chemicals used were of the highest grade available and obtained from commercial sources.
Plant Material and Preparation of Aqueous Extract
Young cactus cladodes of Opuntia ficus indica (2-3 weeks old) were collected from the local area of Elguettar, Gafsa City (Tunisia) on July 2015. They were identified by Dr Lefi El Kadri, Faculty of Sciences of Gafsa, Gafsa University, Tunisia. Cactus cladodes were washed with water, chopped into small pieces (without removing the small leaves and spines), dried at 37 °C and then pressed using a handpress. About 50 g of resulting dry powder was homogenized with 1000 mL of distilled water and centrifuged at 4,000 rotations per minute (rpm) for 15 min at 4 °C to remove any impurity resulting from the extraction process. Opuntia supernatant was stored at -20 °C until use. For biological activities, the supernatant was filtered through Whatman No. 1 filter paper and the filtered solution was lyophilized in a lyophilizer at 4°C. The dry extract was collected and kept at +4°C until further analyses.
Phytochemical Study of O. Ficus Indica
Extraction of phenolic Acids and Flavonoids
The dried powder of cactus (1 g) was mixed with 10 mL of extraction solution (methanol 80%), agitated for 10 min and then centrifuged at 12000 rpm for 5 min. An aliquot of supernatant (0.5 mL) was added to 0.5 mL of acetone and agitated. The homogenate was then centrifuged (12000 rpm for 5 min). A Speed-Vac device was used to dry the homogenate which was then used for HPLC analysis of phenolic acids and flavonoids 22.
Experimental Conditions of High-Performance Liquid Chromatography Mass Spectroscopy
Analyses by liquid chromatography were performed using a Varian Prostar HPLC equipped with a ternary pump (model Prostar 230) and a Prostar 330 diode array detector. The HPLC separation of the active compounds was carried out using C-18 reverse phase HPLC column (Varian, 150 mm × 4.6 mm, particle size 5μm). The mobile phase consisted of water: acetic acid (98:2 v/v) (A) and water: acetonitrile: acetic acid (58:40:2 v/v) (B).The elution gradient used was: 0-80% B for 55 min, 80-100% B for 15 min and 100-0 % for 5 min. The flow rate was 0.9 mL/min and the injection 175 volume was 20 μL. The identifications were performed at 280 phenolic acid may exceed this λ max nm for phenolic acids and at 360 nm for flavonoids based on a comparison between the retention time as well as mass spectra of the peaks in the injected extracts and those of HPLC standard compounds.
Determination of Antioxidant Activities
Ferric Reducing Antioxidant Power (FRAP)
The reducing power of the CCE was determined by assessing its ability to reduce iron (III) as described by the method of Yildirim et al. 23. Briefly, 1.25 mL of phosphate buffer (0.2 M, pH = 6.6) was mixed with 1.25 mL of potassium ferricyanide solution (10 g/L) and one mL of CCE at different concentrations (50–800 mg/mL). The mixtures were incubated at 50° C for 30 min, and then 1.25 mL of 10% (w/v) trichloroacetic acid was added and subsequently centrifuged at 3000 x g for 10 min, followed by mixing 1.25 mL of the supernatant solution with 1.25 mL of distilled water and 0.25 mL of ferric chloride (one g/L). After 10 min, the absorbance was measured at 700 nm. A higher absorbance indicates a higher reducing power. The EC50 value (mg/mL) was the CCE concentration at which the absorbance was 0.5 for the reducing power and was calculated from the graph plotting. Ascorbic acid was used as a standard, and the test was carried out in triplicate.
Metal (Fe2+) Chelating Activity
The chelating ability of Fe2+ ions with CCE aqueous extract was evaluated using the method of Dinis et al. 24. A volume of 0.5 mL of CCE extract at different concentrations ranging from 0.1 to 0.8 mg/mL was added to 1.6 mL demineralized water and 0.5 mL of FeCl2 (2 mM). After 15 min, 0.1 mL ferrozine (5mM) was added to the mixture. After 10 min, the absorbance of the complex (Fe2+/ferrozine) having a red or purple colour was measured at 562 nm. The chelating activity of mixture Fe2+/ferrozine was calculated as:
Chelating power (%) = ((Ac – As)/Ac]) x 100 (1)
Ac is the absorbance of control reaction and as is the absorbance of CCE extract. The EC50 value was defined as the concentration (mg/mL) and was calculated from the graph plotting. Ethylene diamine tetraacetic acid (EDTA) was used as a positive control and the test was carried out in triplicate.
Animals and Treatments
36 male Wistar rats (6-8 weeks old), about 240 g body weight, were purchased from l'Institut Pasteur de Tunis (IPT), Tunisia. They were kept for a two-week adaptation period under the same conditions of temperature (22°C), relative humidity (70 ± 4%), and a dark/light cycle of 12 h. The animals were given standard pellets from SICO, Sfax, Tunisia, and tap water ad libitum. The experimental procedures were carried out according to the general guidelines on the use of living animals in scientific investigations (Council of European Communities, 1986) and approved by the Ethical Committee of Sciences Faculty of Gabes. After the adaptation period, the rats were divided into 4 groups of 9 each. Then, the treatments were carried out as follows:
Group 1 (C): received (0.5mL/ 100 g of body weight b.w.) saline solution (NaCl 0.9%) subcutaneously (Control group).
Group 2 (Cd): rats given cadmium (3.5 mg/kg b.w. daily by subcutaneous injection for 10 weeks) (dissolved in distilled water) and fed on rodent pellets. This concentration was chosen according to previous data 13.
Group 3 (CCE): rats given O ficus indica aqueous extract daily via gavages at 100 mg/kg (b.w.) for 10 weeks.
Group 4 (CCE + Cd): rats given 100 mg/kg (b.w) O ficus indica and then injected with cadmium at a dose of 3.5mg/kg (b.w) daily for 10 weeks.
To explore the protective activity against cadmium hazards, Opuntia ficus indica, at a dose of 100 mg/kg b.w, was administered to the rats by daily oral gavages. This dose was chosen based on earlier reports which revealed its efficiency in preventing Zearalenone- induced genotoxicity 17. The rats were observed for water intake and physical signs of toxicity following treatment, and were weighed daily.
After 10 weeks, rats from each group were rapidly sacrificed by decapitation in order to minimize the handling stress. Blood samples were collected from jugular vein in dried tubes and centrifuged at 1500 g for 15 min at 4°C plasma samples were collected for biochemical analyses to determine calcium phosphorus, ALP and ACP levels , femurs were also quickly excised, and the surrounding muscles and connective tissues were removed. All femur samples were weighed and the femoral length was measured with a digital clipper. Some of them were intended for BMD measurements and the others were stored at -80°C for biochemical analysis. 100 mg of bone samples were taken from the femoral region and homogenized with 2 mL of 0.1M Tris-HCl buffer (pH 7.2) using mortar and pestle according to Ramajayam et al. 25. The homogenates were centrifuged at 10,000 rpm for 30 min, at 4°C and the supernatant was used for biochemical assays.
Biochemical Assays
Protein Quantification
The femur protein contents were measured according Lowry’s method 26 using bovine serum albumin as standard.
Ascorbic Acid Determination
Ascorbic acid determination was performed as mentioned by Jacques-Silva et al. 27. Protein was precipitated in 10 volumes of a cold 4% trichloroacetic acid solution. An aliquot (300 mL) of supernatant adjusted with distilled H2O to a final volume of one mL was incubated at 38 °C for 3 h, then one mL H2SO4 65% (v/v) was added to the medium. The reaction product was determined using color reagent containing 4.5 mg/mL dinitrophenyl hydrazine and CuSO4 (0.075 mg/mL) and the data were expressed as mg of ascorbic acid/g tissue.
Calcium and Phosphorus Levels in Plasma and Femurs
Calcium and phosphorus levels were determined in femurs, after nitric acid mineralization and in plasma using commercial reagent kits (Biocon, Ref 2004).
Plasma Total Alkaline Phosphatase and Acid Phosphatase Levels
The plasma levels of total alkaline phosphatase (ALP) and total tartrate-resistant acid phosphatase (ACP) were analyzed by a colorimetric method (Elitech diagnostics SEES FRANCE, Ref PASL0500; Biomerieux FRANCE, Ref 746419901).
Bone Densitometry
BMD was measured at the femur region by dual-energy X-ray absorbsiometry (DEXA) with a Lunar® DPX-L (USA) densitometer with specific small animal software. All samples measured three times the average record. All densitometrical parameters were determined by the same examiner on the same day. All measurements were carried out with a fine-diameter collimator on the X-ray output. Results are expressed as g/cm2. The BMD can be considered 0 as an indicator of the degree of concentration of mineral within the whole bone 28.
Statistical Analysis
All data were expressed as mean ± standard deviation (SD). Statistical significance was calculated using a one-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. P < 0.05 was considered statistically significant.
Results
HPLC Analysis of Cactus Extracts
The HPLC analysis of cactus cladode extract (CCE) revealed the presence of phenolic acids and flavonoids (Figure 1; Figure 2). The identifications were performed at 280 nm for phenolic acids and at 360 nm for flavonoids based on a comparison between the retention time as well as mass spectra of the peaks in the injected extracts and those of HPLC standard compounds. The compounds that were identified in the CCE were sex phenolic acids: quinic acid, gallic acid, 4-O-caffeoylquinic acid, vanillic acid, coumarin and resveratrol with retention times of 6.820 min, 11.100 min, 22.345 min, 28.1 min, 31.875 min and 36.2 min, respectively. The HPLC elution profile of flavonoids showed five main flavonoids: Catechin, epicatechin, rutin, quercetin and kaempferol with retention times of 23.845 min, 25.512 min, 29.280 min, 34.742 min and 36.461 min, respectively.
Figure 1.HPLC profile of phenolic acids (λ= 280 nm) from O. ficus indica extract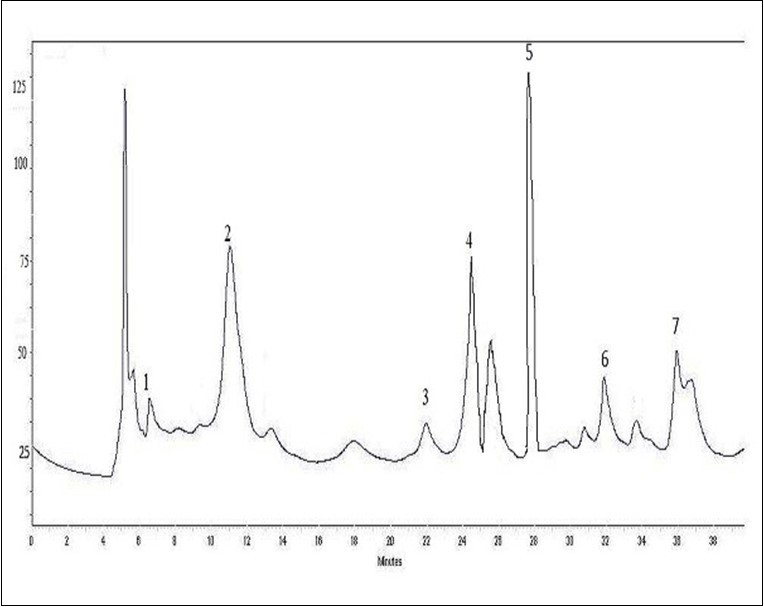
Figure 2.HPLC profile of flavonoids (λ= 360 nm) from O. ficus indica extract
Reducing Power Assay
According to the reducing power assay, it was found that the addition of CCE led to the reduction of Fe3+ to Fe2+ by donating an electron. The effective concentration of CCE EC50 (0.53 ± 0.21 mg/mL) providing 0.5 of absorbance, appeared significantly (p<0.05) lower than that of ascorbic acid (0.39 ± 0.05 mg/mL) used as a positive standards (Table 1).
Table 1. Reducing power and Fe2+ chelating activity of Opuntia ficus indica extract.| Compounds | EC50 reducing power (mg/mL) | IC50 (Fe2+) chelating activity (mg/mL) |
| CCE | 0.36 ± 0.08 | 0.39 ± 0.05 |
| Ascorbic acid | 0.39 ± 0.05 | - |
| EDTA | - | 0.030 ± 0.02 |
Metal (Fe2+) Chelating Activity
The metal chelating activity was evaluated by measuring the formation of the complex ferrozine – Fe2+. Results presented in Table 1 show high-level chelating activity of CCE
(IC50 = 0.39± 0.05 mg/mL) (P<0.05), but was not stronger than the standard EDTA (IC50= 0.030 ± 0.02 mg/mL).
Effects of Treatments on Body Weight, Femur Weight and Length
The results presented in Table 2 show that the total body weight, femur weight, and length were decreased by 26%, 21%, and 5%, respectively, in the Cd-treated group compared to the control group. However, the administration of CCE significantly (P<0.01) increased the body weight of rats compared with the cadmium-treated group. In contrast, CCE alone did not induce any change.
Table 2. Body weight, femur weight, and length of rats.| Parameters studied | C | Cd | CCE | CCE+Cd |
| Initial body weight(g) | 240.69±21.3 | 240.23±19.1 | 236.72 ±19.5 | 238.5±15.85 |
| Final body weight (g) | 361.92± 10.5 | 267.7 ± 11.7* | 372.12 ±20.1++ | 337.75 ±18.4++ |
| Weight of femur (g) | 1.03±0.026 | 0.81±0.014** | 1.02 ±0.02+ | 0.98±0.021+ |
| Length of femur (cm) | 3.18±0.071 | 3.01±0.054* | 3.2±0.08++ | 3.10±0.12++ |
Biochemical Assays
The exposure of rats to 3.5mg/kg of cadmium induced a significant decrease (P<0.01) in calcium (25%) (Figure 3A) and phosphorus (35%) levels compared to control (Figure 3B). Also plasma levels of ACP and ALP bone biomarkers were affected by Cd treatment. In fact, total tartrate-resistant acid phosphatase (ACP), which reflected bone resorption, significantly increased by 54% (P<0.01), and total alkaline phosphatase (ALP), which reflected bone formation, was decreased by 28% (P<0.01) (Figure 4). Also, the results showed indicated that the pretreatment with CCE in combination with cadmium restored all these biomarkers to almost normal values.
Figure 3.Plasma levels of calcium (mg/dL) and phosphorus (mg/dL) of control and experimental rats after 10 weeks of treatment. Values are the mean of 9 measurements ±SD.
Figure 4.Plasma levels of total tartrate-resistant acid phosphatase (ACP) and total alkaline phosphatase (ALP) in the femurs of control and experimental rats after 10 weeks of treatment.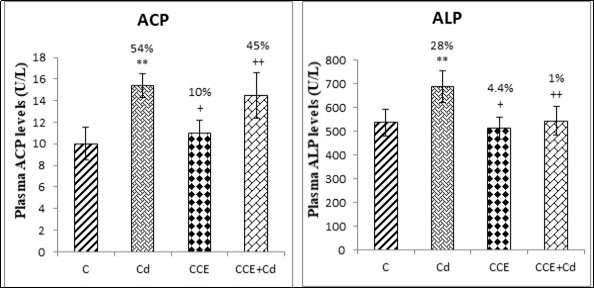
Femur Calcium, Phosphorus and Vitamin Clevels
The exposure of rats to 3.5mg/Kg cadmium altered their bone mineral composition. Indeed, a significant decline (P<0.01) in calcium (32%) and phosphorus (19%) (P<0.05) contents in bone was noted when compared to controls. Besides, vitamin C level decreased by 66% in femur homogenates compared to control group (P<0.01) (Table 3). Treatment with O. ficus indica cladode (100 mg/kg BW) extract alone did not affect these parameters. However, when combined with cadmium, all biochemical levels were restored to their control values.
Table 3. Calcium, phosphorus and vitamin C levels in bone of rats: control, Cd, CCE, and CCE +Cd groups.| Parameters studied | C | Cd | CCE | CCE+Cd |
| Initial body weight(g) | 240.69±21.3 | 240.23±19.1 | 236.72 ±19.5 | 238.5±15.85 |
| Final body weight (g) | 361.92± 10.5 | 267.7 ± 11.7* | 372.12 ±20.1++ | 337.75 ±18.4++ |
| Weight of femur (g) | 1.03±0.026 | 0.81±0.014** | 1.02 ±0.02+ | 0.98±0.021+ |
| Length of femur (cm) | 3.18±0.071 | 3.01±0.054* | 3.2±0.08++ | 3.10±0.12++ |
BMD of the Femur
The BMD of the femur was determined by DEXA (Figure 5). Compared with the control group, the BMD of rats that have been exposed to Cd was significantly (P<0.01) decreased (16%). However, CCE inhibited this effect.
Figure 5.Effects of O.ficus indica extract on bone mineral density (BMD) in the femurs of control and experimental rats after 10 weeks of treatment.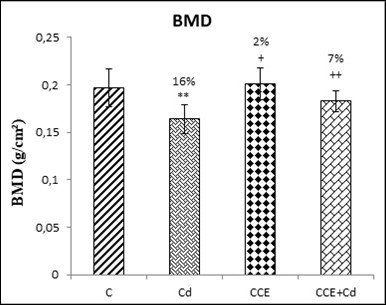
Discussion
Growing attention is paid to the study of plant and bioactive compounds, which may prevent the harmful effects of environmental toxic compounds and combat several human diseases. For this purpose, different types of herbal plants have been re-evaluated and aknowleged as beneficial sources of nutraceuticals 10, 29. In this context, our investigation was carried out to explore the protective effect of CCE on cadmium-induced osteoporosis in adult male Wistar rats. In this purpose, O. ficus indica extract at a dose of 100 mg/kg b.w, was administered to the animals by daily oral gavages. Our Results showed that cadmium induced a significant decrease in the body weight, femur weight and length. This could be explained either by a slight decrease of feed consumption by animals as indicated by Mohamed al. 13 or by malabsorption of nutrients from the gastrointestinal tract in Cd-treated rats as reported by Ball and Chhabra 30. Other findings were recorded by Chen et al. 31 which indicated a decrease in the body weight of rats that were subcutaneously injected with a dosage of 5 mg/kg of Cd. The reduction in femur weight and length in the Cd-treated group observed could be due to the direct action of cadmium on bone development 32.
The bone is a composite material consisting of collagen fibers and hydroxyapatite crystals containing inorganic components mainly calcium and phosphate. It is made up of different cell types: osteoblasts, (bone-forming cells) osteoclasts (bone-resorption cells), osteocytes, and bone lining cells. As a result of its functionality, several metals accumulate in bone 33. In the current study, decreases in calcium and phosphorus, levels compared to controls, were observed. Indeed, the first toxicological reason for Cd-induced bone effects were the clear-cut interference of low level Cd exposure with calcium metabolism 34. Cd exposure decreases calcium and phosphorus absorption, removes calcium with this metal, and delays bone mineralization, which disrupts with the normal ossification 35.
It is well known that the bone is formed and resorbed continuously, starting in the embryo and continuing throughout adult life. This process emerging in adult bone is called bone remodeling. In the current investigation, biochemical markers such as total tartrate-resistant acid phosphatase (ACP), which reflects bone resorption, and total alkaline phosphatase (ALP), which reflects bone formation, increased in the serum. Experimental studies have shown that chronic exposure to Cd reduces mineralization process of skeletal systems, altering their biomechanical properties and rendering them more susceptible to deformity and fracture 36. It is well illustrated that Cd decreases expression of markers of osteoblastic differentiation (Runx2, osteocalcin), of extracellular bone matrix proteins (type I collagen), and of enzymes involved in the mineralization process (alkaline phosphatase-ALP) altering the bone development and mineralization process 7. Other animal studies provide evidence that chronic exposure to Cd decreases bone volume and increases the percentage of tartrate resistant acid phosphatase (ACP) positive cells in subchondral tibial bone 31; the increase in ACP activity would be a signal that osteopenia is provoked by the increase in resorption.
Moreover the study showed that the bone content of calcium and phosphorus were significantly decreased. This weakness indicates that exposure to Cd, results in an increased risk of osteoporosis. Experimental studies have shown that long term Cd exposure induces the inhibition of hydroxyapatite generation and the Cd2+ ions competition with Ca2+ ions for integration into bone 37. Clinical studies have shown that Cd acquired in the body has harmful effects on bone, decreasing bone density and increasing fracture risk and osteoporosis 3.
In our study low level of vitamin C was revealed in Cd-treated group accounting for 61.1 μmoles ascorbic acid/g tissue. Ascorbic acid is a biochemical marker used to evaluate bone function. It is known that vitamin C is an anti stress and a powerful reducing agent. It helps in activating several enzymes and acts as an antioxidant for detoxifying toxic substances 38. Therefore the decrease in the femur total ascorbic acid level in the present study indicated that Cd induced oxidative stress in the bone of rats and that the preserved ascorbic acid was rapidly oxidized in the femur of treated rat.
BMD is an indicator for detection osteoporosis; this parameter gives knowledge concerning the quantity of mineral in bone, which is only one component of bone strength 39. In the present study, the BMD was significantly decreased in Cd-treated rats. Research has shown that calcium failing can reduce BMD and weaken bone strength and long-term calcium deficiency can lead to osteoporosis 40. Moreover, Cd exposure can inhibit the development of bone and reduce BMD because the change in the distribution of elements in the bones 6. This mineral imbalance can cause osteoporosis because of Cd exposure 3. Exposure to Cd may be an important contributing factor to lower BMD and osteopenia, which result in the development of osteoporosis 36.
Phytochemicals has acquired current interest due to their anticipated role in protecting against metabolic diseases. Polyphenols are a group of secondary metabolites which were considered as the most bioactive molecules 41. Therefore, it is important to characterize the phenolic compounds of plant extract. In order to identity the different active compounds of our plant cladodes, we employed HPLC profile analysis. The results show that cactus extracts presented a chemical profile composed of 11 phenolic compounds. Figure 1 and Figure 2 shows that some compounds such as quercetin, kaempferol, isorhamnetin, catechin, quinic acid and coumaric acid were previously reported in O. ficus indica cladodes 42. Alimi et al. 43 confirmed our findings, suggesting that CCE was an important sources of phenolics that preventing oxidative stress, thus reducing the risk of chronic diseases. Further, Guevara-Figueroa et al. 44 determined gallic acid, coumaric acid, protocatechuic as phenolic acids in cactus O. ficus indica cladodes which are similar to our findings. Recent studies proved that phenolic compounds widely distributed in plants are very important because their hydroxyl groups contribute directly to the antioxidant capacity 45. In our investigation, the antioxidant activity of O. ficus indicain vitro is evaluated by several analytical methods such as reducing capacity and metal chelating activity. These assays could be employed together to evaluate the osteoprotective effects of CCE in vivo. The reducing power assay is based on the reduction of Fe3+ to Fe2+ to donate electrons 46. This assay affirmed that the CCE contained a high amount of total phenolics and flavonoids that showed greater reducing power than that of synthetic antioxidant (ascorbic acid). Metal chelating activity is required to be among antioxidant mechanisms since it reduces the concentration of the catalyzing transition metal in lipid peroxidation. Due to its high reactivity, Fe2+ ion is known to be the most important lipid prooxidant transition metal. IC50 value of chelating activity was the concentration of the CCE required to chelate 50% of Fe2+ present in the reaction mixtures. Lower IC50 reflected better chelating activity. Our results indicated that CCE had the highest chelating activity compared with EDTA as a positive standard. This strong antioxidant activity could render CCE an excellent plant protecting cadmium-induced osteoporosis in vivo. This potential could be affected to the presence of phenolic compounds and flavonoids 21, 29. In addition, other researchers have shown that many flavonoids and polyphenols contribute significantly to the antioxidant activity for many fruits and vegetables 17.
The next part of this study was devoted to evaluation of the capacity of CCE to protect against cadmium-induced osteoporosis. Thus, simultaneous administration of CCE (100 mg/kg of b.w.) to cadmium-treated animals partly or entirely inhibited the harmful effects of cadmium in this study. CCE ability to defend the damages induced by cadmium is certainly associated with the presence of diverse antioxidant compounds. In accordance, previous study indicated that the antioxidant properties of CCE are mainly due to vitamins and flavonoids, more particularly vitamin C and quercetin that has been listed to be a highly efficient radical scavenger 18. These findings strongly suggest that CCE extract is highly effective at preventing bone tissue damage. The administration of CCE had a potent protective effect on cadmium-induced damage in rats. Numerous studies using the same extract showed its hepatoprotective, immunoprotective and protective effects against oxidative stress 21, 22. These results suggested that the presence of phenolic compounds in CCE might be the major cause of their significant radical-scavenging activity. These antioxidant properties could render CCE an excellent plant to protect cadmium-induced toxicity in vivo. This potential could be explained by the capacity of CCE to reduce the osteoporotic effects in femur by increasing calcium and phosphorus levels in addition to BMD. Our findings matched with others who reported that administration of CCE had an anti-genotoxic effect occurring in an efficient prevention of chromosomal aberrations and DNA fragmentation against cisplatin induced apoptosis in balb/c mice 47.
Conclusion
In conclusion, this report has proven that (1) Cadmium chloride exposure is a risk factor for osteoporosis; and (2) ‘O. ficusindica’ seems to be a good option. In fact, CCE was found to possess excellent antioxidant activities based on various in vitro and in vivo assays. The different in vitro antioxidant tests proved that the CCE is rich in flavonoids, phenolic compounds. Also, this study demonstrated that CCE could have a protective effect on cadmium-induced osteoporotic damages in our experimental model.
Acknowledgments
This research was funded by the Tunisian Ministry of Higher Education and Scientific Research through the Laboratory of Active Biomolecule Valorisation, the Higher Institute of Applied Biology of Medenine, Mednine, University of Gabes, Tunisia.
References
- 1.Järup L, Akesson A. (2009) Current status of cadmium as an environmental health problem. , Toxicol. Appl. Pharmacol 238, 201-208.
- 2.Yadav R, Archana J, Goyal P K. (2010) Protective action of diltiazem against cadmium induced biochemical changes in the brain of Swiss albino mice. , Ann Neurosci 12, 37-40.
- 3.Wallin M, Barregard G, Sallsten. (2016) Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: the mros sweden study,”. , BJMR: 31(4), 732-741.
- 4.Ercal N, Gurer-Orhan H, Aykin-Burns N. (2001) Toxic metals and oxidative stress: Part 1. Mechanisms involved in metal-induced oxidative damage.Cur.TopMed. , Chem 1, 529-539.
- 5.Akesson A, Bjellerup P, Lundh T. (2006) Cadmium-induced effects on bone in a population-based study of women. , Env. Hea. Persp.; 114(6), 830-834.
- 6.Mellström C. (2016) Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: the MrOS Sweden study. , J. Bone. Miner. Res 31, 732-741.
- 7.Brz'oska M M, Moniuszko-Jakoniuk J. (2005) Disorders in bone metabolism of female rats chronically exposed to cadmium,” Toxicology and Applied Pharmacology;. 202, 68-83.
- 8.Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P et al. (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. , Food Chem 97, 654-660.
- 9.Lee H W, Ko Y H, S B Lim. (2012) Effects of selected plant extracts on anti-oxidative enzyme activities in rats. , Food Chem; 132, 1276-80.
- 10.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne D H. (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruits extracts. , J Food Compos Anal 19, 669-675.
- 11.Kim H J, Kim B S, Lee S J, Kim J S.LeeYR.(2013) Emodin suppresse sinflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology (Oxford). 52, 1583-1591.
- 12.HaridyMAM. (2018) Impacts of fullerene C60 and virgin olive oil on cadmium-induced genotoxicity in rats. , Sci. Total. Env.; 630, 750-756.
- 13.Mohamed S Mustafa1, Omayma M Mahmoud, Hoda H Hussein. (2013) Histological and morphometric effects of CdCl2 and ginger on osteoporosis induced by bilateral ovariectomy in adult albino rats. , Eur. J. Anat; 17(2), 102-114.
- 14.Msaddak L, Siala R, Fakhfakh N, Ayadi M A, Nasri M et al. (2015) Cladodes from prickly pear as a functional ingredient: Effect on fat retention, oxidative stability, nutritional and sensory properties of cookies. , Int J Food Sci Nutr 66, 851-7.
- 15.Stintzing F C, Carle R. (2005) Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol Nutr Food Res. 49, 175-94.
- 16.Awad A B, Chan K C, Downie A C, Fink C S. (2000) Peanuts as a source of betasitosterol, a sterol with anticancer properties. , Nutr Cancer 36, 238-41.
- 17.Zourgui L, Golli E E, Bouaziz C, Bacha H, Hassen W. (2008) Cactus (Opuntia ficus indica) cladodes prevent oxidative damage induced by the mycotoxinzearalenone in Balb/C mice. Food ChemToxicol. 46, 1817-1824.
- 18.Felker P, Del S, Rodriguez C. (2005) Comparison ofOpuntia ficus indicavarieties of Mexican and Argentine origin for fruit yield and quality in Argentina. , J Arid Environ; 60, 405-422.
- 19.Tesoriere L, Butera D, Allegra M, Livrea M A. (2004) Supplementation with cactus pear (Opuntiaficus indica) fruit decreases oxidative stress in healthy humans: a comparative study with vitamin C.Am. , J ClinNutr. ; 80(2), 391-5.
- 20.Zou D, Brewer M, Garcia F. (2005) Cactus pear: a natural product in cancer chemoprevention. , Nutr J; 25, 1-12.
- 21.Ncibi S, Ben Othman M, Akacha A, Krifi M N, Zourgui L. (2008) ficus indicaextract protects against chlorpyrifos-induced damage on mice liver. Food ChemToxicol. 46, 797-80.
- 22.Smida A, Ncibi S, Taleb J, Saad A B, Ncib S et al. (2017) Immunoprotective activity and antioxidant properties of cactus (Opuntiaficusindica) extract against chlorpyrifos toxicity in rats. , Biomed & Pharm 88, 844-851.
- 23.Yildirim A Mavi A, Kara A A. (2001) Determination of antioxidant and antimicrobial activities ofRumex crispusL. , extracts, J. Agric. Food Chem 49, 4083-4089.
- 24.Dinis T C, Maderia V M, Almeida L M. (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. , Arch Biochem Biophys 315, 161-169.
- 25.Ramajayam G, Sridhar M, Karthikeyan S, Lavanya R, Veni S et al. (2007) Effects of Aroclor1254 on femoral bone metabolism in adult male Wistar rats. , Toxicology 241, 99-105.
- 26.Lowry O H, Rosenbrough N J, Randall R. (1951) Protein measurement with the folin phenol reagent. , J BiolChem 193, 265-275.
- 27.Jacques-Silva M C, Nogueira C W, Broch L C, EMM Flores, JBT Rocha. (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. , Pharmaco Toxicol 88, 119-125.
- 28.Ferretti J L, Cointry G R, Capozza R F, Capiglioni R, Chiappe M A. (2001) Analysis of biomechanical effects on bone and on the bone muscle interactions in small animal models. , J. Musculoskelet. Neuron Interact.1: 263-74.
- 29.Zourgui L, Ayed-Boussema I, Ayed Y, Bacha H, Hassen W. (2009) The antigenotoxic activities of cactus (Opuntia ficus-indica) cladodes against the mycotoxin zearalenone in Balb/c mice: prevention of micronuclei, chromosome aberrations and DNA fragmentation. , Food Chem Toxicol 47, 662-667.
- 30.Ball L M, Chhabra R S. (1981) Intestinal absorption of nutrients in rats treated with 2,3,7,8 tetrachlorodi benz-p-dioxin (TCDD). , JTEH 8, 629-636.
- 31.Chen X, Zhu G, Jin T, Gu S, Tan M et al. (2011) Cadmium exposure induced Itai-Itai like syndrome in male rats. , CEJM; 6, 425-434.
- 32.F M El-Demerdash, M I Yousef, F S Kedwany, H. (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective Role of Vitamin E and Beta-Carotene. Food Chem Toxico;. 42, 1563-1571.
- 33.Al Ibrahim T, Abu Tarboush H, Aljada A, Al Mohanna M. (2014) The Effect of selenium and lycopene on oxidative stress in bone tissue in rats exposed to cadmium. Food Nutr. , Sci; 5, 1420-1429.
- 34.Nawrot T S, Staessen J A, Roels H A, Munters E, Cuypers A et al. (2010) Cadmium exposure in the population: From health risks to strategies of prevention. , Biometals; 23, 769-782.
- 35. (2016) Tomaszewska E,Dobrowolski P,Winiarska-Mieczan A,Kwiecień M,Tomczyk A,Muszyński S,Radzki R. , Environ ToxicolPharmacol; 46, 36-44.
- 36.Brz'oska M M, Moniuszko-Jakoniuk J. (2004) Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: Studies on a rat model of human environmental exposure,”Toxico Sci;. 82(2), 468-477.
- 37.Blumenthal N C, Cosma V, Skyler D, LeGeros J. (1995) Walters M. The effect of cadmium on the formation and properties of hydroxyapatite in vitro and its relation to cadmium toxicity in the skeletal system. , Calcif Tissue Int 56, 316-322.
- 38.Suzuki Y. (1990) Synergism of ascorbic acid and glutathione in the reduction of hexavalent chromium in vitro. , Ind. Hea 28, 9-19.
- 39.Friedman A W. (2006) Important determinants of bone strength: beyond bone mineral density. , J Clin Rheumatol 12, 70-7.
- 40.Engstrom A, Michaelsson K, Vahter M, Julin B, Wolk A et al. (2012) Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women,”. , Bone 50(6), 1372-1378.
- 41.Freyschuss B, Ljunggren O, Saaf M, Mellstrom D.and Avenell A,(2007) “Calcium and vitamin D for prevention of osteoporotic fractures,”. , The Lancet 370(9605), 2098-2099.
- 42.Saleem M, Kim H J, Han C K, Jin C.Lee YS.(2006) Secondary metabolites fromOpuntia ficus-indicavar.Saboten. , Phytochemistry 67, 1390-4.
- 43.Alimi H, Hfaiedh N, Bouoni Z, Hfaiedh M, Sakly M et al. (2010) Antioxidant and antiulcerogenic activities ofOpuntia ficus indicaf. inermis root extract in rats. , Phytomedicine 17, 1120-1126.
- 44.Guevara-Figueroa T, Jiménez-Islas H, M L Reyes-Escogido, A G Mortensen, B et al. (2010) Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). , J. Food Compos. Anal 23, 525-532.
- 45.Barros L, A M Carvalho, I C Ferreira. (2010) Leaves flowers, immature fruits and leafy flowered stemsof Malva sylvestris. , Food Chem. Toxicol 48, 1466-1472.
- 46.Yildirim A, Mavi A, Kara A A. (2001) Determination of antioxidant and antimicrobial activities ofRumex crispusL. , extracts. J Agric Food Chem 49, 4083-4089.
Cited by (1)
- 1.Abd El-Aziz Nourhan M., Badr Ahmed N., Farrage Ebtehal A., Darwish Amira M.G., Shehata Mohamed G., 2025, Cadmium toxicity alleviation in rats using lactobacillus-fermented and unfermented opuntia ficus-indica L. juices, Toxicology Reports, 15(), 102089, 10.1016/j.toxrep.2025.102089
