Etodolac, A Preferential COX-2 Inhibitor, does not Inhibit Platelet Aggregation in a Randomized Placebo-Controlled Trial
Abstract
To date, platelet aggregation studies have not been formally evaluated in persons receiving Etodolac, a preferential cyclooxygenase-2 (COX-2) inhibitor. Our purpose was to investigate the influence of Etodolac in therapeutic (analgesic) doses (300 mg every 12h) on platelet aggregation as compared to placebo in healthy volunteers. Platelet aggregation, the primary efficacy variable in this trial, was performed according to the Born method with platelet rich plasma; it was evaluated as maximal platelet aggregation induced by 3 substances (adenosine diphosphate (ADP), epinephrine, collagen); each of these substances was used at 3 different concentrations. No significant difference in platelet aggregation as assessed by Born aggregometry was seen in volunteers treated with etodolac or placebo. Etodolac - applied in regular analgesic doses to volunteers - does not show an inhibitory effect on platelet aggregation and therefore seems an attractive analgesic substance for the perioperative setting.
Author Contributions
Academic Editor: Suofu Qin, Department of Biological Sciences, Allergan, Inc., 2525 Dupont Drive, Irvine, CA 92612-1599.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2013 Norbert Zoller,et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Research into cyclooxygenase inhibition lead to the identification of a constitutional cyclooxygenase activity (cyclooxygenase 1, COX-1), which can be distinguished from a cytokine-induced cyclooxygenase activity (cyclooxygenase 2, COX-2) 1. With the use of cyclooxygenase inhibitors as anti-inflammatory agents and analgesics, unwanted side effects such as mucosal toxicity can occur. It was postulated that such side effects are mainly associated to the effects of COX-1 inhibition 2; and that preferential inhibition of COX-2 would therefore decrease this type of toxicity 3. This lead to the development of preferential COX-2 inhibitors with effective anti-inflammatory and analgesic effects documented 4, 5. As postulated, it became apparent that COX-2 inhibition was indeed associated with a lower degree of mucosal toxicity 6. It was also shown that COX-2 activity is not involved in platelet thromboxane biosynthesis 7 and that selective COX-2 inhibition thus does not effect platelet thromboxane A2 (TxA2) dependent platelet aggregation 8. No such studies were published on the use of Etodolac (Lodine®, Sigma-Tau Pharma AG, Zofingen, Switzerland) in humans so far.
Analgesic drugs that modify enzymatic pathways can influence platelet aggregability and use of non-steroidal anti-inflammatory drugs (NSAIDs) may lead to increased perioperative bleeding risk 9, 10, a side effect seen with COX inhibitors 11. Perioperative bleeding is becoming a more important issue for various reasons, including the increased frequency of regional anesthesia.
Etodolac, a COX inhibitor that was introduced in the 1980s has been marketed world-wide. In 1999, it was identified as a preferential COX-2 inhibitor 12. Which was, however, shown not to be significantly selective towards COX-2 13, 14, 15. However, up to date in medical practice neither an increased risk of bleeding nor an increased risk of cardiovascular side effects had been observed 16, 17, 18.
Only minimal renal side effects have been described 19, 20, 21, with one patient with rheumatoid arthritis developing a membranous nephropathy 22.
A previous study 23 showed that the analgesic effect of a short-term treatment with etodolac after coronary artery bypass operation is superior to tramadol. In that study, a trend toward a faster pain reduction with etodolac as compared with diclofenac was observed, suggesting that the analgesic effect of etodolac in that setting is at least as effective as that of diclofenac. Therefore, it is of interest to know in addition whether Etodolac has any effect on platelet aggregation. The objective of this study was thus to investigate the influence of Etodolac in therapeutic doses on platelet aggregation as compared to placebo in healthy volunteers as no studies on platelet aggregation with the use of Etodolac has been published so far.
Experimental Procedures
Materials and Methods
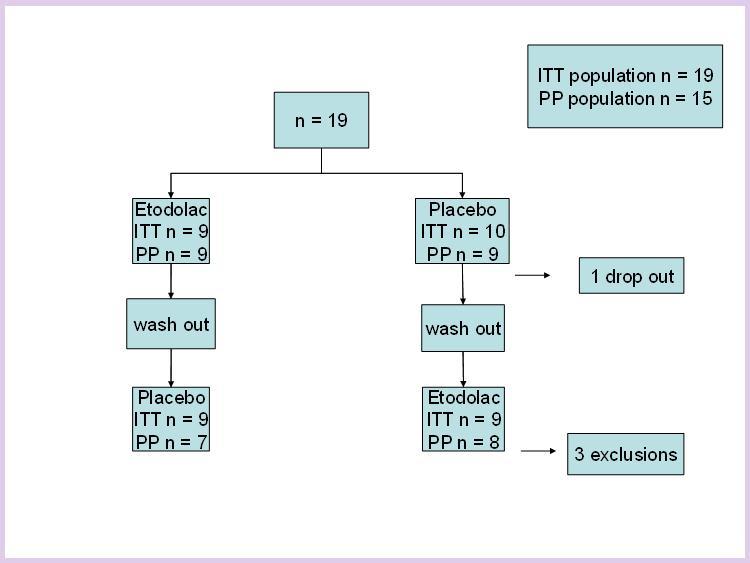
The study was approved by the institutional review board and was registered with the swiss federal regulatory board (Swissmedic 2005DR1357). Volunteers (20 to 50 years of age, table 1) gave written informed consent. This study was designed as an open-label, cross-over, randomized, prospective, monocentric, placebo-controlled study.
Table 1. Demographic data: age, gender| ITT population | PP population | |||
| n | % | n | % | |
| Total | 19 | 100 | 15 | 100 |
| Age (years) 20-29 | 6 | 31.6 | 4 | 26.7 |
| 30-39 | 4 | 21.1 | 3 | 20 |
| 40-49 | 9 | 47.4 | 8 | 53.3 |
| Total | 19 | 100 | 15 | 100 |
| Gender female | 11 | 57.9 | 8 | 53.3 |
| male | 8 | 42.1 | 7 | 46.7 |
Determination of Sample Size
For the primary variable, a normal distribution was assumed. We anticipated a mean aggregation of 80% of the normal (± 15%) in the placebo group. A mean of 65% (± 15%) (i.e. a difference of 15%) was considered as a clinically significant deviation. The power calculation was performed as specified for AB/BA (two-stage cross-over) trials. To reach a power of 80%, a sample size of 12 subjects was thus needed.
The sequence of treatment allocation with randomization was blockwise (5 volunteers per block).
In-/Exclusion Criteria
Exclusion criteria included the use of NSAIDs in the last 14 days before study entry; known hypersensitivity to any active or inactive ingredient of the study medication or placebo; history of allergic reactions to acetyl salicylic acid or other NSAIDs; use of platelet inhibiting medication during the last 30 days before study entry; diagnosis of malignancy; any history of cardiovascular, respiratory, renal, hepatic, haematological, neurological or psychiatric pathologies; a history of gastric and / or duodenal ulcers or other gastrointestinal bleeding; inflammatory bowel diseases; history of thromboses; history of bleeding, alone or in conjunction with NSAID use; concomitant medication (except oral contraceptives); women of child-bearing potential not willing to subject to a medically accepted method of contraception; pregnant or nursing women; and participation in another clinical trial in the 30 days preceding the study.
Before enrolment, a complete medical history was taken and a physical examination was performed. Laboratory parameters evaluated at this point were liver function, renal parameters and complete blood count.
Treatment
All subjects received Etodolac batch number 5LX002 (Lodine®) (300 mg) and placebo batch number 1104 (containing 53 mg lactose monohydrate, 55 mg Avicel® PH 101, 5 mg AcDiSol® and 2 mg sodium stearyl fumarate).
The number of tablets of the study medication handed out to and returned by the patients was documented. Study medication (Etodolac or placebo) was dispensed for 7 days; the volunteers were asked to take the study medication as prescribed every 12 h and to return to the clinic after 7 days for visit 2. With each visit at the clinic, the volunteers had to return the blisters used for the study medication, thus allowing the investigators to document the number of pills apparently consumed by the volunteer. The investigators also questioned the volunteers if they had consumed the pills taken from the blisters. At this point, the volunteers were also queried about the occurrence of adverse events (AE) and serious adverse events (SAE); all prior examinations were repeated. Thereafter, a 7 day washout period started. After the washout period, all examinations were repeated again (visit 3) and the volunteers received the alternate study medication (placebo or Etodolac) for 7 days. After another week, all examinations were repeated once more and the study was concluded (visit 4).
Aggregation Studies
Platelet aggregation studies were performed in platelet rich plasma according to the Born method 24 on an APACT 4004 aggregometer (Haemochrom Diagnostica GmbH, Essen, Germany) using low, medium and high concentrations of ADP, epinephrine and collagen as inductors. Platelet aggregometry was performed according to the local standard operating procedure, including adjustment of platelet count to 250 G/l with autologous plasma.
Outcome Parameters
The primary efficacy variable in this trial was the extent of platelet aggregation during treatment with Etodolac or placebo. Platelet aggregation was evaluated as percent of maximal amplitude of platelet aggregation induced by 3 substances (adenosine diphosphate (ADP), epinephrine, collagen) with series of platelet aggregation responses at 3 different “dose levels”, i.e. concentrations (ADP and epinephrine: 1.25µM, 5µM, 10µM; collagen: 1.25µg/ml, 5µg/ml, 10µg/ml).
As the measurement method remained the same in all instances, the model was performed with the mean value of the measurements of the 3 substances at each point in time.
Statistical Analysis
All statistical tests were interpreted on the 5% significance level.
A complete analysis according to an AB/BA-trial with 2 treatments (active agent and placebo) and 2 treatment periods was performed. The influence of the active agent on platelet aggregation as compared to placebo was tested using ANOVA (analysis of variance).
The data entry was performed in a database of Microsoft Office Access 2003, Version 11.0, using forms with plausibility checks. No double data entry was done.
The data analyses were performed using SPSS 13.0 software (descriptive analysis) and R 2.0.1 software (statistical analysis).
Analyses were done for the ITT (intention to treat; all randomized subjects with at least one intake of the trial medication) and the PP (per protocol; excluding subjects that had either violations of the inclusion/exclusion criteria, or violations of the study protocol, or dropped out of the study prematurely) population.
Results
The ITT population comprised 19 subjects (11 females).
The PP population included 15 subjects (8 females). Mean age ± standard deviation (SD) in this population was 37.1 ± 9.5 years.
No abnormal laboratory value was observed in any volunteer during the study.
Of the ITT population comprising 19 subjects; one volunteer was taken off study after visit 1 due to possible adjudication bias (this person was referred by the sponsor); three volunteers had protocol violations, leaving a PP population of 15 subjects. All 3 volunteers with protocol violations finished the study but were not included in the PP population. Two of the protocol violations were related to mistakes with the intake of the study medication, one was related to a substantial delay in the time of blood sampling.
There were no significant differences in the results of the ITT population as compared to the PP population. In both populations, no difference between the effect of Etodolac or placebo on platelet aggregation studies was observed, neither with high nor low or medium concentrations of ADP, epinephrine or collagen (p= 0.1998). There was also no evidence that the sequence (Placebo followed by Etodolac versus Etodolac followed by Placebo) had any effect on platelet aggregation (p= 0.8314).
Results comparing the influence of placebo or Etodolac on platelet aggregation as induced by epinephrine, collagen or ADP are displayed in Figure 1 and Figure 2, Figure 3 (for clarity, only the results of the highest concentrations used for each substance are displayed). It can be seen that there is no significant difference in inducible platelet aggregation when volunteers are treated with Etodolac or placebo.
Figure 1.The results of platelet aggregation with collagen as an inducer (here: 10µg/ml) before Etodolac, after Etodolac, after washout and after placebo are displayed.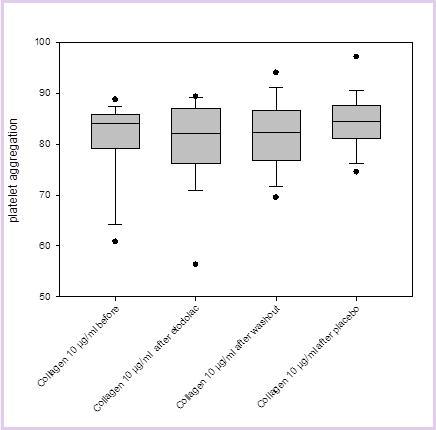
Figure 2.The results of platelet aggregation with ADP (adenosine diphosphate, here 10µM) as an inductor before Etodolac, after Etodolac, after washout and after placebo are displayed.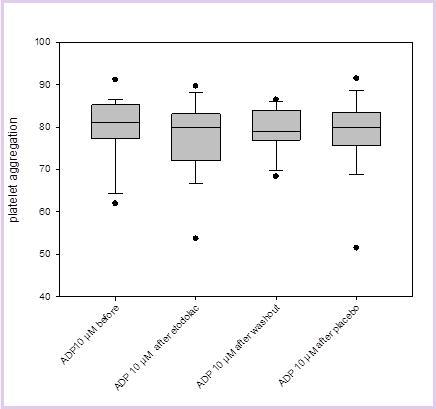
Figure 3.The results of platelet aggregation with epinephrine as an inducer (here: 10µM) before Etodolac, after Etodolac, after washout and after placebo are displayed.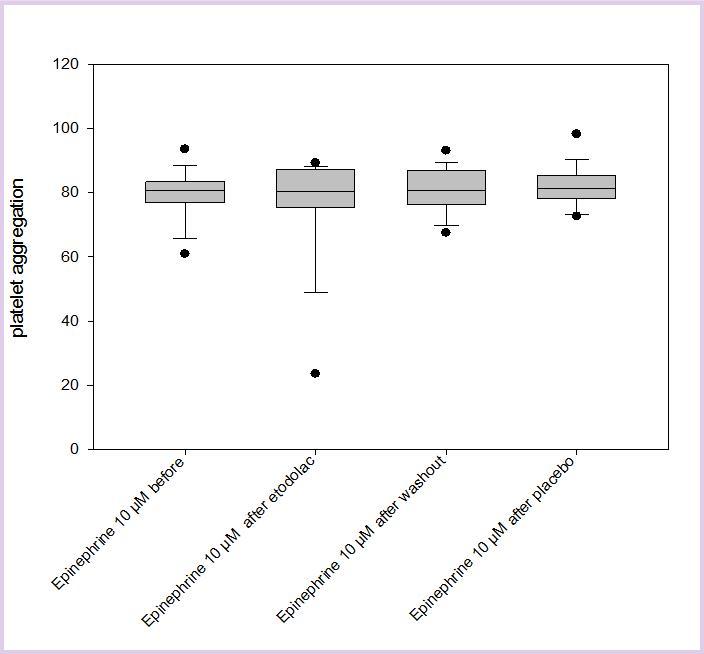
Only one adverse event (abdominal discomfort) according to the study protocol was observed; it was judged to be possibly related to the study medication, but not to be clinically relevant. Of the four volunteers that were not included in the PP population, two each were in the placebo (cross over to etodolac) and the etodolac (cross over to placebo) group. The ITT population comprised 9 volunteers in the etodolac group and 10 volunteers in the placebo group (see figure 4).
Discussion
The main finding of our study is that etodolac at regular analgesic doses (300 mg every 12 h for 7 days) has no inhibitory effect on platelet aggregation performed according to the Born method when compared to placebo.Until now, it has been assumed that NSAIDs with non-selective inhibition of both the COX-1 and COX-2 enzymes increase the risk of bleeding due to their COX-1 inhibitory effect, resulting in reduced platelet aggregation 25. Given that there is no relevant influence on platelet aggregation, selective inhibition of COX-2 supposedly reduces the risk of associated bleeding problems. In fact, a number of studies have demonstrated that selective COX-2 inhibition do not affect platelet aggregation (or affect it to a significantly lesser extent) 26, 27, 28, 29, 30, but this has not been undisputed 31. COX-2 inhibiting drugs might be appropriate tools to provide a perioperative analgesic approach without the risk of increased bleeding. These observations with a seemingly well tolerated drug 16, 17 might be particularly interesting since Rofecoxib, a selective COX-2 inhibitor, was withdrawn from the market due to its cardio-vascular and renal side effects 32, reigniting the debate on the safety of selective COX-2 inhibitors 33, 34, 35.
Others have shown that neither short- nor long-term exposure to etodolac was associated with cardionegative or -protective effects as compared to ibuprofen, rofecoxib and celecoxib 18.
As NSAIDs are frequently used postoperatively for analgesic treatment 36, 37, side effects from these drugs are important when assessing postoperative morbidity. Most NSAIDs have been shown to influence platelet aggregation not only in vitro, but also to a clinically potentially relevant extent 9, 10. As shown in this study and in contrast to most other NSAIDs, etodolac has no influence on platelet aggregation ex vivo. This parallels the post marketing experience available today 19, 38, 39.Limitations of our study are the small sample size and the fact that it was performed in volunteers; however, the population size was based on stringent sample size calculation and there was no difference in results between the ITT population and the PP population.
Evaluation of the platelet COX-1 and -2 pathways can be achieved using various inductors of platelet aggregation.
When investigating the influence of COX inhibitors, the use of arachidonic acid can result in a too sensitive assay, as described by Becker et al. 40. In that study, the use of arachidonic acid led to the observation that 97% of all patients showed no aggregation after COX inhibition, prohibiting the statistical evaluation of these results. Such an approach was also suggested by Akagi et al. 41, who found no additional effect of etodolac on top of aspirin in an animal model. Therefore, we used ADP, collagen and epinephrine for aggregation induction in three different (low, medium and high) concentrations.
The fact that we used only the Born method as the “gold standard” for platelet aggregation studies might be perceived as a potential study weakness, since other methods are also available. However, clinical studies evaluating different platelet aggregation study methodologies in parallel in patients undergoing coronary stent implantation 42 did not reveal any predictive differences between the different assays. And the ADP – induced platelet aggregation according to Born showed a better ability to detect differences in platelet aggregation when compared with the VASP assay 43.
As data from this study (and in accordance with animal studies) show that etodolac has no influence on platelet aggregation in healthy volunteers at regular doses, it seems that this substance might be an important addition to the analgesic armentarium in the perioperative setting. Others have shown that etodolac is efficacious in postoperative pain control in cardiac surgery 23, where platelet aggregation inhibition is an important issue with regard to catheters used for analgesic therapy. In the aforementioned animal study 41, no additional effect of etodolac on top of aspirin in inhibiting platelet aggregation was found. It would be interesting to further investigated this combination in a clinical study.
We believe that our study adds to the body of knowledge suggesting that etodolac is indeed advantageous in situations where nonsteroidal analgesics are desirable but where adequate hemostasis is a concern at the same time.
Conclusion
Our study shows that etodolac - applied in regular analgesic doses to volunteers - does not show an inhibitory effect on platelet aggregation and therefore seems to be an attractive analgesic substance for the perioperative setting.
Acknowledgements
Sigma-Tau Pharma AG, Switzerland, payed for the study drug, placebo and for the diagnostic procedures performed at the Center for Laboratory Medicine. No further conflicts of interest are to be reported.
The current affiliation of Norbert Zoller is “Geriatrische Klinik St. Gallen”.
References
- 1.Mitchell J A, Akarasereenont P, Thiemermann C, Flower R J, Vane J R. (1993) Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. , Proc Natl Acad Sci U S A 90(24), 11693-7.
- 2.Seibert K, Masferrer J L. (1994) Role of inducible cyclooxygenase (COX-2) in inflammation. , Receptor 4, 17-23.
- 3.Crofford L J. (1997) COX-1 and COX-2 tissue expression: implications and predictions. , J Rheumatol Suppl 49, 15-9.
- 4.Ehrich E W, Schnitzer T J, McIlwain H, Levy R, Wolfe F. (1999) Effect of specific COX-2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. Rofecoxib Osteoarthritis Pilot Study Group. , J Rheumatol 26, 2438-47.
- 6.Goldstein J L, Silverstein F E, Agrawal N M, Hubbard R C, Kaiser J. (2000) Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitor. , Am J Gastroenterol 95, 1681-90.
- 7.Patrignani P, Sciulli M G, Manarini S, Santini G, Cerletti C. (1999) COX-2 is not involved in thromboxane biosynthesis by activated human platelets. , J Physiol Pharmacol 50, 661-7.
- 8.McAdam B F, Catella-Lawson F, Mardini I A, Kapoor S, Lawson J A. (1999) Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. , Proc Natl Acad Sci U S A 96, 272-7.
- 9.Kallis P, Tooze J A, Talbot S, Cowans D, Bevan D H. (1994) Pre-operative aspirin decreases platelet aggregation and increases post-operative blood loss--a prospective, randomised, placebo controlled, double-blind clinical trial in 100 patients with chronic stable angina. , Eur J Cardiothorac Surg 8(8), 404-9.
- 10.Scharf R E.(2012Nov)Drugs that Affect Platelet Function. Semin Thromb Hemost. , Epub2012Oct30 38(8), 865-83.
- 11.Ng K F, Lawmin J C, Tsang S F, Tang W M, Chiu K Y.(2009Jun)Value of a single preoperative PFA-100 measurement in assessing the risk of bleeding in patients taking cyclooxygenase inhibitors and undergoing total knee replacement. , Br J Anaesth. Epub2009May2 102(6), 779-84.
- 12.Warner T D, Giuliano F, Vojnovic I, Bukasa A, Mitchell J A. (1999) Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. , Proc Natl Acad Sci U S A 96, 7563-8.
- 13.Giuliano F, Ferraz J G, Pereira R, G de Nucci, Warner T D.(2001Aug24)Cyclooxygenase selectivity of non-steroid anti-inflammatory drugs in humans: ex vivo evaluation. , Eur J Pharmacol 426(1), 95-103.
- 14.Kawai S, Nishida S, Kato M, Furumaya Y, Okamoto R.Comparison of cyclooxygenase-1 and -2 inhibitory activities of various nonsteroidal anti-inflammatory drugs using human platelets and synovial cells. , Eur J Pharmacol.(1998april17) 347(1), 87-94.
- 15.Riendeau D, Charleson S, Cromlish W, Mancini J A, Wong E et al.Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. , Can J Physiol Pharmacol.(1997Sep) 75(9), 1088-95.
- 16.Graham D J, Campen D, Hui R, Spence M, Cheetham C.2005Feb(5-11) Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. , Lancet.; 365(9458), 475-81.
- 17.Bonnel R, Karwoski C.OPDRA Postmarketing Safety Review“, Thrombotic Vascular Events. , FDA Memorandum
- 18.Motsko S P, Rascati K L, Busti A J, Wilson J P, Barner J C. (2006) Temporal relationship between use of NSAIDs, including selective COX-2 inhibitors, and cardiovascular risk. Drug Saf. 29(7), 621-32.
- 19.Jones R A. (1999) Etodolac: An overview of a selective COX-2 inhibitor. , Inflammopharmacology 7(3), 269-75.
- 20.Svendsen K B, Bech J N, Sørensen T B, Pedersen E B.A comparison of the effects of etodolac and ibuprofen on renal haemodynamics, tubular function, renin, vasopressin and urinary excretion of albumin and alpha-glutathione-S-transferase in healthy subjects: a placebo-controlled cross-over study.Eur. , J Clin Pharmacol.(2000Aug) 56(5), 383-8.
- 21.Lee A, Cooper M G, Craig J C, Knight J F, Keneally J P.(2007april18)Effects of nonsteroidal anti-inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database Syst Rev. (2): CD 002765
- 22.Sugimoto T, Aoyama M, Kikuchi K, Sakaguchi M, Deji N. (2007) Membranous nephropathy associated with the relatively selective cyclooxygenase-2 inhibitor, etodolac, in a patient with early rheumatoid arthritis. , Intern Med. (Epub2007Jul2) 46(13), 1055-8.
- 23.Immer F F, Immer-Bansi A S, Trachsel N, Berdat P A, Eigenmann V. (2003) Pain treatment with a COX-2 inhibitor after coronary artery bypass operation: a randomized trial. Ann Thorac Surg. 75, 490-5.
- 25.Schrör K. (1997) Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost. 23, 349-56.
- 26.Hegi T R, Bombeli T, Seifert B, Baumann P C, Haller U. (2004) Effect of rofecoxib on platelet aggregation and blood loss in gynaecological and breast surgery compared with diclofenac.BrJ Anaesth. 92, 523-31.
- 27.Weaver A L. (2001) Rofecoxib: clinical pharmacology and clinical experience. Clin Ther. 23, 1323-38.
- 28.Munsterhjelm E, Niemi T T, Ylikorkala O, Neuvonen P J, Rosenberg P H. (2006) Influence on platelet aggregation of i.v. parecoxib and acetaminophen in healthy volunteers. BrJ Anaesth. 97, 226-31.
- 29.Leese P T, Recker D P, Kent J D. (2003) The COX-2 selective inhibitor, valdecoxib, does not impair platelet function in the elderly: results of a randomized controlled trial. J Clin Pharmacol. 43, 504-13.
- 30.Silverman D G, Halaszynski T, Sinatra R, Luther M, Rinder C S. (2003) Rofecoxib does not compromise platelet aggregation during anesthesia and surgery. CanJ Anaesth. 50, 1004-8.
- 31.Hernandez M R, Tonda R, Pino M, Serradell M, Arderiu G. (2004) Evaluation of effects of rofecoxib on platelet function in an in vitro model of thrombosis with circulating human blood. Eur J Clin Invest. 34, 297-302.
- 32.Rocha J L, Fernandez-Alonso J. (2001) Acute tubulointerstitial nephritis associated with the selective COX-2 enzyme inhibitor, rofecoxib. , Lancet 357, 1946-7.
- 33.James M J, Cleland L G. (2004) Applying a research ethics committee approach to a medical practice controversy: the case of the selective COX-2 inhibitor rofecoxib. J Med Ethics. 30, 182-4.
- 34.Weir M R, Sperling R S, Reicin A, Gertz B J. (2003) Selective COX-2 inhibition and cardiovascular effects: a review of the rofecoxib development program. , Am Heart J 146, 591-604.
- 35.Whelton A, Fort J G, Puma J A, Normandin D, Bello A E. (2001) SUCCESS VI Study Group. Cyclooxygenase-2--specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. , Am J Ther 8, 85-95.
- 36.Jirarattanaphochai K, Jung S.Nonsteroidal antiinflammatory drugs for postoperative pain management after lumbar spine surgery: a meta-analysis of randomized controlled trials. , J Neurosurg Spine.(2008Jul) 9(1), 22-31.
- 37.Novelli G P, Casali R, Cecconi P.Nonsteroidal anti-inflammatory drugs (NSAID) in the treatment of postoperative pain. Minerva Anestesiol.(1990Jul-Aug),56(7-8). 345-7.
- 38.Schattenkirchner M. (1990) An updated safety profile of etodolac in several thousand patients. , Eur J Rheumatol Inflamm 10(1), 56-65.
- 39.Neustadt D H.Double blind evaluation of the long-term effects of etodolac versus ibuprofen in patients with rheumatoid arthritis. J-Rheumatol-Suppl.( Feb1997). 4717-22.
- 40.Becker D M, Segal J, Vaidya D, Yanek L R, Herrera-Galeano J E.Sex differences in platelet reactivity and response to low-dose aspirin therapy. , JAMA.(2006 Mar22) 295(12), 1420-7.
- 41.Akagi Y, Nio Y, Shimada S, Aoyama T. (2011) Influence of nonsteroidal anti-inflammatory drugs on the antiplatelet effects of aspirin in rats. Biol Pharm Bull. 34(2), 233-7.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
Journal of Pharmacological and Toxicological Methods (2023) Crossref
Y. Koshman, Aimee L. Bielinski, Brandan M. Bird, J. R. Green, K. Kowalkowski et al. - Journal of pharmacological and toxicological methods (2023) Semantic Scholar
SSRN Electronic Journal (2022) Crossref