Abstract
Lenalidomide is a second generation immunomodulatory agent and a potent analogue of thalidomide that is FDA approved mainly for the treatment of multiple myeloma (MM) and transfusion-dependent anemia due to low or intermediate-1- risk myelodysplastic syndromes (MDS) associated with 5q deletion among other indications. Through its action on the immune system, lenalidomide alters the production of different cytokines ultimately resulting in immune activation against tumors. This immune activation may lead to collateral immune toxicities like fever, angioedema, Stevens-Johnson syndrome, tumor flare and others. Here we report a case of lenalidomide-induced high grade fever in a patient with MM and we summarize the literature about the physiology of such reaction and how to mitigate this adverse event.
Author Contributions
Academic Editor: Angela Pia Cazzolla, University of Bari, Italy.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Ghada ElGohary, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Case Report
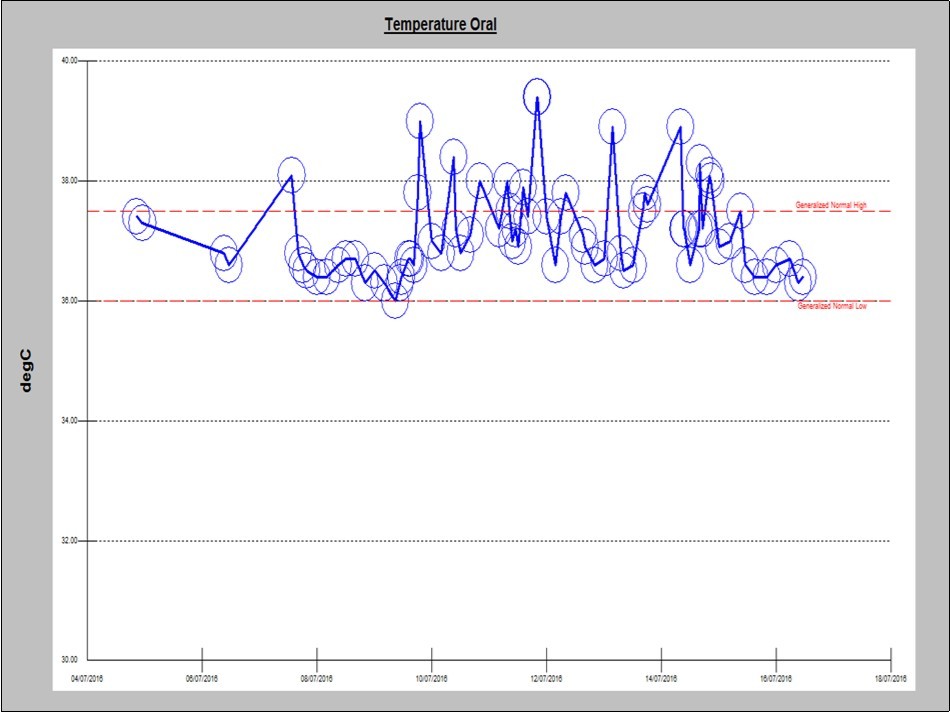
A 59 year-old-woman with newly diagnosed IgG kappa MM presented to the emergency department with fever and generalized body aches one day after starting her first cycle of therapy with lenalidomide, bortezomib and dexamethasone. She denied any shortness of breath, cough, runny nose, dysuria, diarrhea, abdominal pain, and headache or neck stiffness. Physical examination was unremarkable except for fever (temperature of 39° C) and tachycardia. Patient was started on broad spectrum antibiotics and admitted for workup. Infectious workup included repeated blood and urine cultures, chest x-ray, nasal swab for viruses and all were negative. Her complete blood count and differential was within normal limits. Patient remained febrile for 5 days (Figure 1), despite escalating and changing her antimicrobial therapy. After six days of hospitalization, antibiotics and lenalidomide were discontinued. Fever subsided within few hours and patient was then discharged in stable condition. The patient was then treated with cyclophosphamide, bortezomib and dexamethasone and underwent autologous hematopoietic cell transplant after achieving a very good partial remission. The patient refused maintenance therapy post-transplant and relapsed 2 years after transplant. Upon relapse, patient was treated with low dose lenalidomide (10 mg daily), bortezomib and dexamethasone and tolerated this regimen well and later on lenalidomide dose was escalated to the standard dose of 25 mg daily
Discussion
Lenalidomide is a 4-amino-glutamyl analogue of thalidomide, modified in a way to minimize the neurologic side effects and to improve the efficacy of thalidomide. It is a second generation immunomodulatory agent approved by FDA for clinical use mainly in myelodysplastic syndromes with deletion of chromosome 5q, multiple myeloma among other cancers1. As an immunomodulatory drug it affects both the cellular (T and NK cells activation) and humoral components of the immune system, which results in down regulation (TNF-α, IL-6, IL-8) or up regulation (IL-2 and IFN-γ) of various cytokines and growth factors and eventually leads to changes in the tumor microenvironment2. Fever is frequently encountered while caring for patients with hematologic malignancies. Lenalidomide induced fever is not infrequent, and may be easily overlooked because it is not appropriately reported or highlighted in the literature.
Cancer patients may develop fever for multiple reasons (infections, thrombosis, drugs…). Drug fever is a febrile response that coincides temporally with the administration of a drug and disappears after discontinuation of the offending agent. It is usually suspected when no other causes of fever can be identified, often after antimicrobials have already been started. Fever can be the sole manifestation of an adverse drug reaction in 3-5 % of cases3,4. The mechanisms of drug fever are multiple and, in many cases, are poorly or incompletely understood3,4. The lenalidomide package insert ranks fever as one of the most common adverse reactions that can affect more than 20% of the patients on lenalidomide. The mechanism of lenalidomide-induced fever remains unclear however it is well known that lenalidomide can increase the production of pro-inflammatory cytokines (IL-6 and TNF-α) that cause elevation of CRP5, enhances immunological synapse formation and upregulates CD80 on tumor cells which leads to T cell activation and rapid cytokine release6,7, and these in turn may cause fever. In a published case report a patient with MM was started on lenalidomide and 4 days later he was admitted with high grade fever and elevated CRP, his pre-treatment plasma interleukin-6 and tumor necrosis factor levels were normal and these increased significantly after starting lenalidomide. The fever resolved spontaneously after stopping lenalidomide7. Tumor flares have been reported after using lenalidomide for the treatment of chronic lymphocytic leukemia8, and this might be another mechanism of lenalidomide induced fever, however the exact mechanism remains unclear9. The management of these febrile reactions consists of holding the medication until resolution of the symptoms; patient can be rechallenged at a lower dose followed by escalation as tolerated.
Conclusion
In summary, fever due to lenalidomide therapy seems to be frequent but not highlighted in the medical literature. Fever is not reported with the use of thalidomide, the first generation immunomodulatory; however fever incidence in the phase III registration trial for pomalidomide was 27% 10. As such this side effect might be a class side effect related to the potency of the different generations. Whether the incidence of fever correlates with the activity of these agents remains unanswered, keeping in mind that neither the degree of T cell activation in vitro nor the severity of the cytokine release syndrome correlated with the activity of lenalidomide in lymphomas 11. Medical teams might consider holding the medication without rushing to costly investigations and without starting antibiotherapy especially in the absence of infectious symptoms. Physicians should be aware of this potential association given the increased frequency of lenalidomide usage. Further studies are needed to clarify whether this phenomenon influences the clinical outcomes.
References
- 1.Kotla V, Goel S, Nischal S, Heuck C, Vivek K et al. (2009) Mechanism of action of lenalidomide in hematological malignancies. , Journal of hematology & oncology 2(1), 36.
- 2.Chanan-Khan A A, Cheson B D. (2008) Lenalidomide for the treatment of B-cell malignancies. , Journal of Clinical Oncology 26(9), 1544-1552.
- 3.BYH Thong, Tan T C.Epidemiology and risk factors for drug allergy. , British journal of clinical 71(5), 684-700.
- 4.Riedl M A, Casillas A M. (2003) Adverse drug reactions: types and treatment options. , American family physician 68(9), 1781-1790.
- 5.Ferrajoli A, Lee B-N, Schlette E J, O'Brien S M, Gao H et al. (2008) Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. , Blood 111(11), 5291-5297.
- 6.Quach H, Ritchie D, Stewart A K, Neeson P, Harrison S et al.Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. 24(1), 22.
- 7.Nakamura N, Kanemura N, Shibata Y, Matsumoto T, Mabuchi R et al.Lenalidomide-induced cytokine release syndrome in a patient with multiple myeloma. , Leukemia & 55(7), 1691-1693.
- 8.Moutouh-de Parseval LA, Weiss L, DeLap R J, Knight R D, Zeldis J B. (2007) Tumor lysis syndrome/tumor flare reaction in lenalidomide-treated chronic lymphocytic leukemia. , Journal of Clinical Oncology 25(31), 5047-5047.
- 9.Morita Y, Shimada T, Yamaguchi T, Rai S, Hirase C et al.Cytokine profiles in relapsed multiple myeloma patients undergoing febrile reactions to lenalidomide. , International journal of 94(6), 583-584.