Biodegradation of Malathion Using Pseudomonas stutzeri(MTCC 2643)
Abstract
Pesticides are applied in agricultural fields for controlling pest population to achieve crop protection. But they cause damage to nontarget organisms and affect the quality of environment including water, air and soil. The present study has been designed to test the efficiency of Pseudomonas stutzerion the degradation of malathion. The bacterial strain was subjected to 50, 100, 150 and 200 ppm of malathion in minimal broth for 30 hours and changes in orthophosphate levels, pH and turbidity were monitored for every six hours. Efficiency of free and immobilized cells were compared for orthophosphate release. Influence of different sugars on degradation was also compared. Degradation of 150 ppm of malathion was confirmed with UV-Visible spectrophotometric analysis and HPLC analysis. The data were subjected to two way analysis of variance and the results are discussed.
Author Contributions
Academic Editor: Larance Ronsard, National Institute of Immunology, New Delhi-110067
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Vaishali S, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Pesticides are used to prevent the plants from pests. They are most commonly abundant environmental chemicals present in soil, water, food and so on, due to their extensive applications in agriculture. Pesticides applied in the soil affect the fertility and productivity of soil and sometimes cause pollution in aquatic ecosystems by entering into them from the agricultural fields making water unsuitable for human consumption. They persist in the environment for a long period of time, which causes severe problems to the environment and also to human beings. Therefore, it is inevitable to discover appropriate strategies to overcome the problems caused by pesticides 1, 2, 3, 4, 5.
The use of bioremediation technology to degrade organic contaminants including hazardous pesticides has achieved a great deal of attention in recent years. Research has revealed that microbial degradation process to detoxify pesticide contaminants can be effectively used to overcome the pollution problems 6, 7, 8. Bacterial strains with the capacity of degrading several pesticides have been isolated from soil. They include metamitron- degrading Rhodococcus sp. 9, chlorpyrifos-degrading Flavobacterium sp. 10 and iprodione-degrading Arthrobacter sp. 11. Pseudomonas putida strain was isolated, which was able to utilize diclofop- methyl as a source of carbon and energy 12.
Malathion is a non-systemic pesticide and one of the world's most widespread general-purpose organophosphates. It exhibits high selective toxicity and is mostly used in the control of sucking and chewing insects on fruits and vegetables and vector mosquitoes and flies. It is available in emulsifiable concentrate, wettable powder, and ultra low volume formulations. It is an acetyl cholinesterase inhibitor, and so acetylcholine accumulates causing over-stimulation and nervous collapse in human beings. It is rapidly absorbed through the gastrointestinal tract, skin, mucous membranes, and lungs 13, 14, 15.
Malathion persists in soil with reported field half lives of 1 to 25 days. In aquatic systems, the major degradation pathway for malathion is through hydrolysis. Hydrolysis occurs when the malathion molecule chemically reacts with water. It has been demonstrated that the rate of hydrolysis increases with increasing temperature and alkalinity of the water. Half lives for malathion in water ranges from 1.5 days to 21 weeks. Photolysis, or degradation by light, may provide significant breakdown in surface waters exposed to large amounts of solar radiation. It was found that malathion was not used as a primary source of carbon for the bacteria. However, evidence of enzymatic by-products indicated that the bacteria used malathion as a secondary source of carbon. These enzymatic hydrolysis by-products included mono-carboxylic acid and di-carboxylic acid derivatives of malathion 16, 17, 18, 19, 20, 21. In the present study, an attempt has been made to determine the malathion degrading ability of Pseudomonas stutzeri strain (MTCC 2643) obtained from IMTECH, Chandigarh, Punjab, India.
Materials and Methods
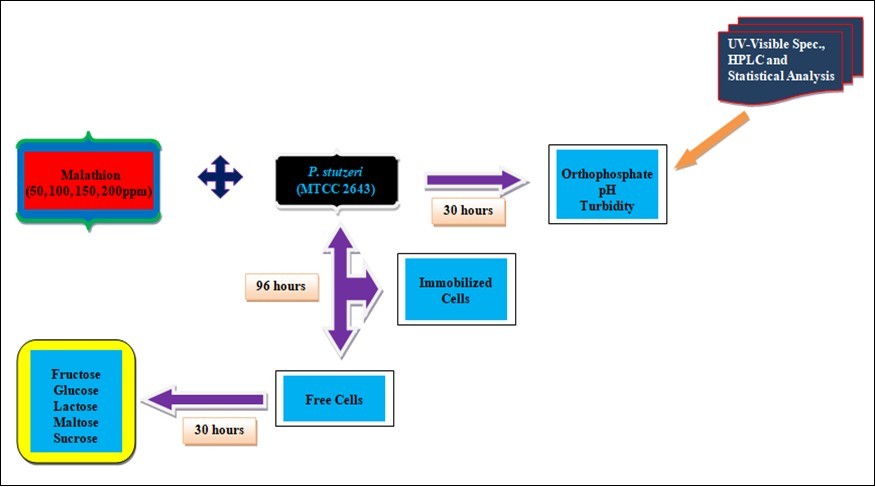
Pesticide Used
Malathion, an orthophosphate pesticide was selected for the present study based on its broad range of application in the agricultural fields and present market trends.
Preparation of Sample
Pseudomonas stutzeri strain (Microbial Type Culture Collection (MTCC) 2643) obtained from Institute of Microbial Technology (IMTECH), Chandigarh, Punjab, India was inoculated as one ml (109 cells) from the logarithmic phase of the pure culture grown in nutrient broth into minimal broth containing different concentrations of commercial grade (raw pesticide) malathion (50, 100, 150 and 200 ppm). Based on growth assays, it was inferred that the selected bacterial strain could tolerate malathion only up to 200 ppm. Hence the above-mentioned concentrations were selected. The flasks were incubated at room temperature and the samples were then subjected for the estimation of orthophosphate. All the values calculated represent the means of three observations.
Orthophosphate Estimation
1 ml of sample was taken in a flask and 1 ml of ammonium molybdate and 3 drops of stannous chloride solution were added and kept for 10 minutes for the development of blue colour and the absorbance was recorded in a colorimeter at 650 nm. Distilled water blank was subjected in a similar manner.
Similarly the standard phosphorus solution of different strengths was processed and standard curve was plotted between absorbance and the concentrations of standard phosphorus solution. The orthophosphate content of the sample was deduced by comparing its absorbance with the standard curve.
pH Measurement
pH was analyzed every 6 hours up to 30 hours of treatment for the samples from different concentrations of malathion using pH meter and readings were recorded.
Turbidity Measurement
Growth was measured as turbidity at 600 nm in 6 hours interval for 30 hours.
Supplementation of Sugars
The efficiency of pesticide degrading ability of the bacterial strain was tested by providing different carbon sources like fructose, glycerol, lactose, maltose and sucrose of 1% concentration in minimal medium containing 150 ppm concentration of malathion. 150 ppm was chosen for further experiments based on the changes in orthophosphate released, pH and turbidity. The flasks were incubated at 37°C and orthophosphate released was estimated every 6 hours up to 30 hours.
Immobilization of Cells
The seed cultures of the strain were grown in nutrient broth and the cells were harvested by centrifugation at 10,000 rpm for 10 minutes and the cells were washed and suspended in 0.1% NaCl. Then 3.5% of sodium alginate was added to the cell suspension and mixed thoroughly without forming any air bubble in the slurry. The slurry containing the cells was extended as drops through a tube (2 mm diameter) into 4% CaCl2 solution. The drops formed into spherical beads of 2 mm size. The gel beads were kept in 4% CaCl2 solution at 5°C for about an hour for complete gelation. Then the beads were washed with sterile distilled water and used for malathion degradation study 22.
UV-Visible Spectrophotometric Analysis
The samples from 150 ppm concentration of malathion were centrifuged at 6 hours interval for 30 hours and the clear supernatant was used for spectral analysis. The clear supernatant was scanned from 200 to 600 nm in a spectrophotometer (Elico SL: 159) and analysed for specific absorption in the spectrum.
High Pressure Liquid Chromatographic (HPLC) Analysis
The samples from 150 ppm concentration of malathion before and after 30 hours of treatment period were subjected to HPLC analysis by UV detection.
Statistical Analysis
Two way analysis of variance (ANOVA) was performed on the factors like orthophosphate released, turbidity, and pH for the two variables namely treatment period and malathion concentration using MS-Excel package. Variations due to treatment period or malathion concentration were considered statistically significant when the calculated F value was greater than the table value at 5% level.
Results and Discussion
Malathion, one of the world's most wide-spread general-purpose organophosphorus insecticides with high selective toxicity is mostly used for the control of sucking and chewing insects and for controlling mosquitoes and flies. Microbial cleavage is responsible for the degradation organophosphorus compounds. To make sound pest management decisions, pesticide users and resource managers should have an understanding of the fate of pesticides in the environment. Hydrolytic cleavage of organophosphate bond is considered as the initial step in the metabolism of organophosphates. But the hydrolytic reaction did not supply energy required for the growth of the organisms and only the degradation products from these pesticides appear to serve as energy for growth and proliferation of microorganisms 23, 24. Orthophosphate released during the degradation of malathion at different concentrations of malathion (50, 100, 150, and 200ppm) by P. stutzeri was analysed in the present study (Figure 1). At 0 hr the level of orthophosphate was 7 μg/ml. Initially the orthophosphate concentration was increasing gradually and there was a decline after 18 hours. At the end of 18 hours, orthophosphate released was high in the degradation of 150ppm concentration of malathion. So, 150ppm concentration of the malathion was found to be optimum for the degradation of pesticide and it was selected for further analysis.
Figure 1.Orthophosphate released during the degradation of Malathion by P. stutzeri
Effective degradation of malathion by P. stutzeri was carried out at the pH range of 6-8 (Figure 2). With the increase in incubation period, pH was decreasing indicating the release of acidic intermediates. The alteration in the pH during the growth of P. stutzeri in malathion amended minimal medium proved not only the breakdown of malathion but also the formation of acidic intermediates. It has been reported that the alteration of pH in the medium from 7.2 to 3.0 during the growth of Serratia marcescens in malathion amended medium confirmed the breakdown of malathion and formation of acidic intermediates 25. Similarly, the pH of the assay mixtures showed the change in pH from 7.2 to 6.0 - 6.1 at the end of three hours, which confirmed the breakdown of malathion and the formation of acidic intermediates 26. It is observed that, initially, at 0 hour, the inoculated cell population was low and then started increasing slowly until 18 hours and P. stutzeri entered into the phase of positive acceleration. P. stutzeri utilized malathion as a sole source of carbon and phosphorus for their growth.
Figure 2.Changes in pH during the degradation of Malathion by P. stutzeri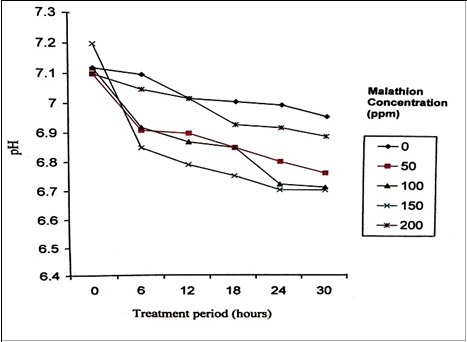
Turbidity measurement (Figure 3) divulges that there was a significant increase in the growth during the treatment period until 24 hours which indicates that the organisms effectively utilized the pesticide as the sole source of carbon and phosphorus. At 0 hour the absorbance was 0.45. The bacterial cells in log phase denote that the substrate conversion is at its maximum. Decline phase was not achieved even after 18 hours of incubation indicating that the nutrients were still available for the cells to grow and other environmental conditions are also favourable. Decrease in cell density was observed after a logarithmic phase. It has been reported that Pseudomonas sp. has the ability to grow in medium containing malathion (35-220 mg/L). However, the optimum concentration which supported normal bacterial growth during 24 hours was found to be 120 mg/L malathion. When compared to control, a significant increase in bacterial population was noticed at low concentration, while at high concentration lag phase increased 12, 27.
Figure 3.Turbidity during the degradation of Malathion by P. stutzeri
The use of immobilized enzymes in large-scale industrial processes has recently progressed from the stage of theoretical consideration into reality. Immobilized pesticide hydrolyzing enzymes conceivably could be used for pesticide detoxification creating a continuous or discontinuous, economical, safe method, usable in either large or small-scale systems 28. The technique of immobilizing cells is advantageous because the immobilized cells serve as self-proliferating and self-regenerating biocatalysts. Immobilization prevents washout of cells and allows a high cell density to be maintained. The catalytic stability is often improved upon immobilization, as microorganisms may tolerate and degrade high of toxic compounds than that of their counter parts 29. Immobilized pesticide-degrading bacteria can be used on a large scale for pesticide detoxification 30. Immobilized cells released orthophosphate with a constant, stable and gradual increase but in the case of free cells the concentration of orthophosphate declined after 12 hours of treatment. At 0 hour the level of orthophosphate released was 7 μg/ml (Table 1). Immobilized P. stutzeri were able to degrade malathion gradually with constant increase in the cell population when cornpared to free cells where degradation proceeds at a faster rate and there was a decline in cell population after 72 hours of treatment period. Immobilized enzyme kinetics was approximately 50% slower than that of the free enzyme, but there was no significant difference in the effect of pH and temperature on the activity of immobilized and free enzyme(s)31. Immobilized pesticide-degrading cell system is in the form of an extracorporal shunt which may fill a medical void in the treatment of pesticide poisoning cases 32.
Table 1. Two way analysis of variance for the factors with the variables, treatment period and malathion concentration| Factor | Source ofVariation | SS | df | MS | CalculatedF - value | Table valueat 5% level | Level ofsignificance |
| Orthophosphate released | Treatment Period | 155.4 | 4 | 38.86 | 3.562 | 3.007 | Significant P‹ 0.05 |
| Malathion Concentration | 1648.6 | 4 | 412.0 | 37.76 | 3.007 | Significant P‹ 0.05 | |
| pH | Treatment Period | 0.176 | 4 | 0.044 | 12.18 | 2.866 | Significant P‹ 0.05 |
| Malathion Concentration | 0.345 | 4 | 0.069 | 19.09 | 2.711 | Significant P‹ 0.05 | |
| Turbidity | Treatment Period | 1.74 | 4 | 0.434 | 57.41 | 3.007 | Significant P‹ 0.05 |
| Malathion Concentration | 0.393 | 4 | 0.098 | 13.01 | 3.007 | Significant P‹ 0.05 |
Supplementation of carbon sources to the minimal medium enhanced degradation process where P. stutzeri utilized sucrose and fructose for degradation of malathion. The level of orthophosphate was 7 μg/ml at 0 hour (Figure 4). Addition of 1% concentration of different carbon sources enhanced the degradation of malathion with the release of high levels of orthophosphate. Lactose, sucrose and fructose showed effective degradation of malathion. It has been reported that when fructose was the growth substrate, the rate of demeton-S-methyl (Organophosphate) consumption by Corynebacterium glutamicum was greater than the rate of consumption when either acetate or glucose was the growth substrate 33. Addition of 1% concentration of different carbon sources indicated disaccharides enhancing the degradation of chloropyrifos with the release of orthophosphate, total phosphorus and increasing the activity of acid phosphatase when compared with that of other carbon sources 34.
Figure 4.Orthophosphate released during the degradation of 150ppm Malathion by free and immobilized cells of P. stutzeri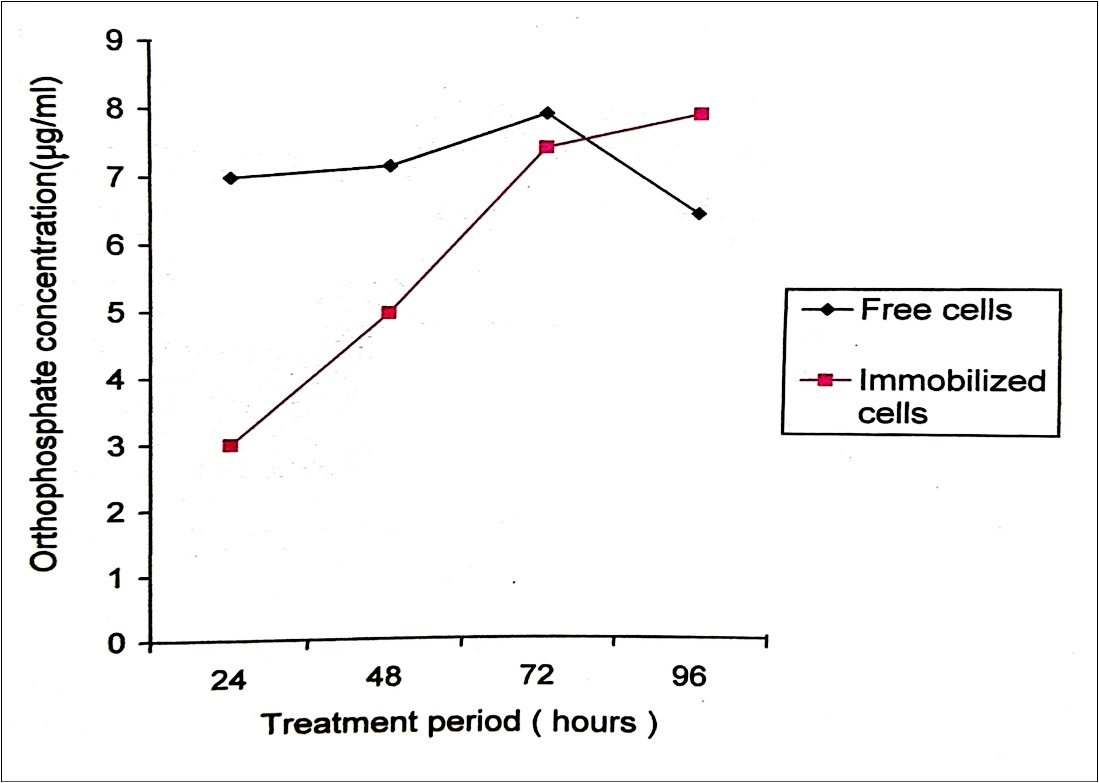
Kannan and Vanitha 25 reported that the assay mixture containing mineral salts medium at the end of three hours showed two characteristic absorption regions i.e. one at 230-235nm and the other one at 240-245nm in spectrometric analysis. The degradation of malathion by P. stutzeri was clearly observed that there was an increase in absorbance till 12 hours and gradually declined in absorbance with increase in incubation period (Figure 5). The specific absorption peak of malathion was observed at 200-300 nm in minimal medium that extended from UV to visible region but the spectrum obtained after 6 hrs of incubation showed absorption peaks with the total disappearance of peaks specific for malathion. HPLC is a most powerful method used for pesticide analysis for a long period of time 35. In the present study, HPLC was performed to confirm the bacterial degradation of malathion at 30 hours of treatment. The retention time for standard malathion was 3.390 minutes and after the degradation of malathion by P. stuzeri it was 3.413 minutes (Figure 6). The peak value in Figure 7 shows the presence of an intermediate compound and the difference in the retention time indicates the occurrence of intermediate compound formed during the degradation of malathion. This indicates that degradation was carried out by P. stutzeri. In the earlier studies, malathion degradation by Bacillus licheniformis was analysed by HPLC 36. HPLC analysis showed that Brevibacillus sp. and Bacillus cereus were able to degrade 36.22% and 49.31% of malathion, respectively, after 7 days of incubation. Both strains were able to survive well in the medium containing malathion concentration up to 0.15%. 37. P. stutzeri was capable of degrading malathion upto the concentration of 200 ppm in a significant manner. Further studies related with the genes responsible for the degradation of malathion in P. stutzericould be candy for developing an efficient recombinant strain through r-DNA technology.
Figure 5.Orthophosphate released during the degradation of 150ppm Malathion by P. stutzeri when supplemented with various sugars of 1% concentration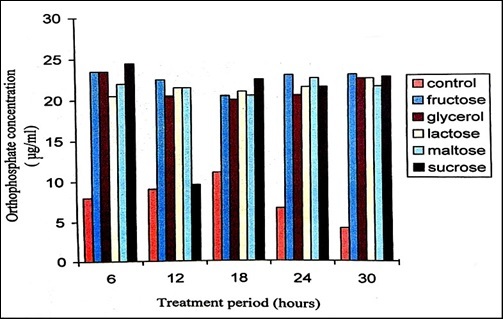
Figure 6.UV-Visible absorption spectrum taken during the degradation of 150ppm Malathion by P. stutzeri
Figure 7.HPLC analysis report for 150ppm Malathion degradation by P. stutzeri (a) before and (b) after 30 hours of treatment period 
Pesticide degradation ability of tested samples indicated statistical difference in degradation process in sediment and in water 38. Table 1 exhibits the two way ANOVA for the factors such as orthophosphate released during degradation by P. stutzeri, pH and turbidity with the variables, treatment period and malathion concentration. Variations due to treatment period and malathion concentration were statistically significant at 5% level.
Pseudomonads exhibit diverse catabolic pathways that enable them to metabolize several low molecular weight compounds, like chlorinated aliphatic hydrocarbons such as phenoxyalkanoic acid herbicides. They are known for their capacity to degrade phenolic compounds and other aromatic substances and therefore are an ideal choice as the bacteria to be used for degradative biotechnologies 39, 40. In the present study, orthophosphate levels, pH and turbidity changes and the modifications in UV Visible absorption spectrum and HPLC peaks indirectly indicated the degradation of malathion by the chosen bacterial strain. Monitoring malathion concentration in the medium can facilitate the prediction of percentage of degradation. The bacterial strain can be effectively employed in bioremediation programmes either by immobilization or supplementation with sugars.
Conclusion
The selected strain was able to degrade malathion effectively upto 200 ppm and the immobilized cells can perform better than that of free cells. In the case of live cells supplementation with sucrose and fructose exhibited better results.
Acknowledgement
The authors thank the authorities of The American College, Madurai, Tamil Nadu, India, for the facilities and encouragement.
References
- 1.Botías C, Basley K, Nicholls E, Goulson D. (2019) Impact of pesticide use on the flora and fauna of field margins and hedgerows”. The Ecology of Hedgerows and Field Margins. 90-109.
- 2.Hossain L, Rahman R, M S Khan. (2017) Alternatives of pesticides”. In: Pesticide residue infoods.Springer,Cham. 147-165.
- 3.Mahmood I, Imadi Shazadi, Gul K, Hakeem A, KR. (2016) Effects of pesticides on environment”. In: Plant, soil andmicrobes.Springer,Cham. 253-269.
- 4.Abubakar Y, Tijjani H, Egbuna C, C O Adetunji, Kala S et al. (2020) Pesticides, History, and Classification”. In: Natural Remedies for Pest, Disease and WeedControl.AcademicPress. 29-42.
- 5.Nazir T, Khan S, Qiu D. (2019) . , Biological Control of Insect Pest”. In: Pests-Insects, Management, Control. Intech Open
- 6.G O Adams, P T Fufeyin, S E Okoro, Ehinomen I. (2015) Bioremediation, biostimulation and bioaugmention: a review”. , Int. J.Envt.Bioremed.Biodegrad 3(1), 28-39.
- 7.K T Semple, K J Doick, L Y Wick, Harms H. (2007) Microbial interactions with organic contaminants in soil: definitions, processes and measurement”. , Environmental 150(1), 166-176.
- 8.Gavrilescu M. (2005) Fate of pesticides in the environment and its bioremediation”. Engineering in Life Sciences.5(6): 497-526.
- 9.Fang H, Xu T, Cao D, Cheng L, Yu Y. (2016) Characterization and genome functional analysis of a novel metamitron-degrading strainRhodococcussp. MET via both triazinone and phenyl rings cleavage”. Scientific reports. 6-32339.
- 10.Dhanya M S. (2014) Advances in microbial biodegradation of chlorpyrifos. , Journal of Environmental Research and Development 9(1), 232-241.
- 11.Campos M, P S Karas, Perruchon C, E S Papadopoulou, Christou V et al. (2017) Novel insights into the metabolic pathway of iprodione by soil bacteria”.Environmental Science and Pollution Research.24(1):. 152-163.
- 12.Jilani S, M A Khan. (2004) Isolation, characterization and growth response of pesticides degrading bacteria”. , J. Biol. Sci 4(1), 15-20.
- 13.Thompson T S, Treble R G, Magliocco A, Roettger, Eichhorst J C. (1998) Case study: fatal poisoning by malathion”.Forensic science international. 95(2), 89-98.
- 14.V K Patil, David M. (2008) Behaviour and respiratory dysfunction as an index of malathion toxicity in the freshwater fish,Labeorohita(Hamilton)”.Turkish Journal of fisheries and aquatic sciences. 8(2), 233-237.
- 15.P B Tchounwou, A K Patlolla, C G Yedjou, P D Moore. (2015) Environmental exposure and health effects associated with Malathion toxicity”. Toxicity and Hazard of Agrochemicals. 51, 2145-2149.
- 16.M A Brown, M X Petreas, H S Okamoto, T M Mischke, R D Stephens. (1993) Monitoring of malathion and its impurities and environmental transformation products on surfaces and in air following an aerial application”.Environmental science & technology. 27(2), 388-397.
- 17.K V Ragnarsdottir. (2000) Environmental fate and toxicology of organophosphate pesticides”. , Journal of the Geological Society 157(4), 859-876.
- 18.J O Lalah, S O Wandiga. (1996) The persistence and fate of malathion residues in stored beans (Phaseolus vulgaris) and maize (Zea mays)”. Pesticide science. 46(3), 215-220.
- 19.D G Karpouzas, B K Singh. (2006) Microbial degradation of organophosphorus xenobiotics: metabolic pathways and molecular basis”. Advances in microbial physiology.51: 119-225.
- 20.Abdullah A R, Bajet C M, Matin M A, Nhan D D, Sulaiman A H. (1997) Ecotoxicology of pesticides in the tropical paddy field ecosystem’. Environmental Toxicology and Chemistry:. , An International Journal 16(1), 59-70.
- 21.Odukkathil G, Vasudevan N. (2013) Toxicity and bioremediation of pesticides in agricultural soil”.Reviews. in Environmental Science and Bio/Technology.12(4): 421-444.
- 22.Elakkiya M, Prabhakaran D, Thirumarimurugan M. (2016) Methods of cell immobilization and its applications”.Methods.5(4):. 211-216.
- 23.P, B J, N M Deshpande, S. (2004) Biodegradation of organophosphorus pesticides”. Proceedings -Indian National Science Academy Part B 70(1), 57-70.
- 24.B K Singh, Walker A. (2006) Microbial degradation of organophosphorus compounds”. FEMS microbiology reviews. 30(3), 428-471.
- 25.Kannan V, Vanitha V. (2005) Influence of assay medium on degradation of malathion by Serratia marcescens”. , Indian Journal of Biotech 4(2), 277-283.
- 26.K M Zeinat, A H Nashwa, A I Mohamed, E N Sherif. (2008) Biodegradation and detoxification of malathion by Bacillus thuringiensis MOS-5”. , Australian Journal of Basic and Applied Sciences 2(3), 724-732.
- 27.D J Cork, J P Krueger. (1991) Microbial transformations of herbicides and pesticides”. , Adv. Appl. Microbiol 36, 1-66.
- 28.Nannipieri P, J M Bollag. (1991) Use of enzymes to detoxify pesticide-contaminated soils and waters”. , Journal of environmental quality 20(3), 510-517.
- 29.Efremenko E, Peregudov A, Kildeeva N, Perminov P, Varfolomeyev S. (2005) New enzymatic immobilized biocatalysts for detoxification of organophosphorus compounds”. , Biocatalysis and Biotransformation 23(2), 103-108.
- 30.S R Caldwell, F M Raushel. (1991) Detoxification of organophosphate pesticides using an immobilized phosphotriesterase from Pseudomonas diminuta”. Biotechnology and bioengineering. 37(2), 103-109.
- 31.D M Munnecke. (1977) Properties of an immobilized pesticide-hydrolyzing enzyme”. , Appl. Environ. Microbiol 33(3), 503-507.
- 32.Gao Y, Y B Truong, Cacioli P, Butler P, I L Kyratzis. (2014) Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles”. Enzyme and microbial technology. 54, 38-44.
- 33.Girbal L, J L Rols, N D Lindley. (2000) Growth rate influences reductive biodegradation of the organophosphorus pesticide demeton by Corynebacterium glutamicum”. , Biodegradation 11(6), 371-376.
- 34.Thengodkar R R M, Sivakami S. (2010) Degradation of Chlorpyrifos by an alkaline phosphatase from the cyanobacterium Spirulina platensis”. , Biodegradation 21(4), 637-644.
- 35.W H Leong, S Y Teh, M, Nadarajaw T, Zabidi-Hussin Z et al. (2020) Application, monitoring and adverse effects in pesticide use: the importance of reinforcement of good agricultural practices (GAPs)”. , Journal of Environmental Management 260, 109987.
- 36.Khan S, Zaffar H, Irshad U, Ahmad R, A R Khan et al. (2016) Biodegradation of malathion by Bacillus licheniformis strain ML-1”. , Archives of Biological Sciences 68(1), 51-59.
- 37.Singh B, Kaur J, Singh K. (2012) Biodegradation of malathion by Brevibacillus sp. strain KB2 and Bacillus cereus strain PU”. , World J. Microbiol. Biotechnol 28, 1133-1141.
- 38.Kuranchie-Mensah H, S M Atiemo, L D Palm, Blankson-Arthur S, A O Tutu et al. (2012) Determination of organochlorine pesticide residue in sediment and water from the Densu river basin. , Ghana”. Chemosphere 86(3), 286-292.
Cited by (10)
This article has been cited by 10 scholarly works according to:
Citing Articles:
Tissue and Cell (2026) Crossref
Mamdouh B Eldesoqui, Eman M Embaby, Rania A Fouad, Yara M Alrajhi, Zeinab A Mohammed et al. - Tissue & Cell (2025) Semantic Scholar
Tissue and Cell (2025) OpenAlex
Mauricio Rosso-Pinto, Vicente Vergara-Flórez, J. Marrugo-Negrete - Revista Brasileira de Engenharia Agrícola e Ambiental - Agriambi (2023) Semantic Scholar
Revista Brasileira de Engenharia Agrícola e Ambiental (2023) OpenAlex
Revista Brasileira de Engenharia Agrícola e Ambiental (2023) Crossref
W. Madbolly, Manal F. Abdelall, Sanaa S. Zaki, Hanan A. Nour El-Din, Mona I. Fahd et al. - Journal of Scientific Research in Science (2022) Semantic Scholar
Journal of Scientific Research in Science /Journal of Scientific Research in Science (2022) OpenAlex
Charles Frank Saldaña Chafloque, G. E. A. Aycho - (2021) Semantic Scholar
Manglar (2021) OpenAlex
Pesticide Biochemistry and Physiology (2021) OpenAlex
Pesticide Biochemistry and Physiology (2021) Crossref
G. Kumar, S. Lal, P. Bhatt, R. Ram, A. K. Bhattacherjee et al. - Pesticide Biochemistry and Physiology (2021) Semantic Scholar
