A Systematic Review of the use of Bupropion for Hypoactive Sexual Desire Disorder in Premenopausal Women
Abstract
Objective:
To assess the efficacy of bupropion therapy for hypoactive sexual desire disorder (HSDD) in women.
Methods:
A systematic review was performed utilizing the standard databases. Data were abstracted for study quality, characteristics, and outcomes. Due to the small number of studies and lack of consistently reported outcomes, a meta-analysis was not performed.
Results:
Two studies (289 women) met inclusion criteria. While one study had low risk of bias, the other had areas of high risk of bias. Both trials reported improvement in sexual function domains with treatment ranging from 12 weeks to 112 days.
Conclusions:
Despite two trials demonstrating benefit with bupropion treatment for premenopausal women with HSDD, the evidence is limited and not of adequate quality to recommend the therapy. More trials are needed in this area.
Author Contributions
Academic Editor: Serap Simavli, Pamukkale University School of Medicine, Department of Obstetrics and Gynecology, Denizli, Turkey
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Alyssa J.Bolduan, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Female sexual dysfunction is a prevalent problem worldwide with reported rates of 10-30% 1, 2, 3. Hypoactive sexual desire disorder (HSDD) is a common form of sexual dysfunction affecting females more often than males at rates of 30% and 15%, respectively 2, 4. HSDD is defined in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) as “the persistent or recurrent deficiency or absence of sexual fantasies/thought, and/or desire for sexual activity that causes personal distress or interpersonal difficulties”5. It is not uncommon for women with HSDD to also have concomitant sexual arousal and/or orgasmic disorders 6. Additionally, HSDD and low sexual desire in general are believed to be underestimated by health care providers and are associated with decreased relationship satisfaction and distress for many women and their partners 1, 7. Furthermore, impaired sexual desire has been shown to be associated with depressed mood. While studies have shown improvement in sexual function with relief of depressive symptoms, additional investigation into the psychological and physiological effects of antidepressants has provided evidence of antidepressant-induced sexual dysfunction as an adverse effect of many of these medications 8. Despite ongoing research into female sexual dysfunction and HSDD, the exact etiology of these disorders is yet to be determined.
There have been many trials conducted to determine the efficacy of various therapies in the treatment of HSDD. Decreased androgen levels have been shown to be associated with a decline in libido. Testosterone therapy (oral, transdermal patch, or topical) has been investigated through randomized controlled trials (RCTs) indicating statistically significant improvement in sexual desire 1. Testosterone transdermal patches have been approved for HSDD treatment in Europe. However, the U.S. Food and Drug Administration did not approve testosterone therapy for the treatment of HSDD due to the unknown long term adverse events (Moynihan 2004). In addition to testosterone therapies, investigations of genital vasodilators, dopaminergic therapies, estrogen therapies, and melanocortin receptor-acting bremelanotide have been conducted 1, 8. However, despite these investigations, there continues to be no FDA approved treatment for HSDD in the United States.
Bupropion is a centrally-acting medication that is currently approved for use in the treatment of major depressive disorder, seasonal affective disorder, and smoking cessation (Bupriopion package insert 2014). The exact mechanism of action of bupropion has yet to be determined, however, it is believed to act centrally on both dopaminergic and norepinephrine systems with no serotonergic action 8. In addition to its role in mood improvement, bupropion has been shown to be effective in the reversal of SSRI-induced sexual dysfunction when supplemented to current therapy as well as a substitution therapy 9, 10, 11. In addition to treatment of iatrogenic sexual dysfunction, study results indicate prosexual effects through the use of bupropion 12, 13. The objective of this study was to systematically review the data regarding bupropion treatment for HSDD treatment and to perform a meta-analysis to synthesize the trial results to determine if bupropion is effective treatment for HSDD.
Methods
This was a systematic review of randomized controlled trials. We searched the terms “psychological sexual dysfunction,” “physiological sexual dysfunction,” “libido,” “hypoactive sexual desire disorder,” and “bupropion” using the following computerized databases: MEDLINE (1948-present), MEDLINE In-Process, and The Cochrane Database of Clinical Trials (4th quarter 2013). We limited the search to articles reporting randomized controlled trials in humans and duplicate trial entries were excluded. The search was performed in November 2013. (Figure 1) To ensure completeness of our search, we cross-referenced additional reviews concerning HSDD and uses of bupropion. We additionally read abstracts of titles that appeared applicable and obtained full text articles of abstracts that were relevant for the topic. Articles were read and reviewed by two authors (D.H. and A.B.), who then extracted data independently from those studies that met the criteria for inclusion in this review. Disagreement between the two authors was resolved by consensus. There were no published abstracts alone that were relevant to this review or with sufficient data provided for inclusion in data analysis.
Figure 1.Flow diagram of trial search for the systematic review.
We included RCTs that reported comparison between bupropion and placebo. Trials needed to include premenopausal women diagnosed with idiopathic HSDD. We excluded studies that only investigated bupropion without a control group. We also excluded trials using bupropion for treatment of medication-induced sexual dysfunction. Interventions were grouped into categories of control and bupropion, in which control was the placebo treatment. We recorded but did not control for differences in medication dose and schedule. We utilized the Cochrane risk of bias assessment tool (high risk, unclear, or low risk) (Higgins 2011). We included all articles and planned to perform a sensitivity analysis based on the results of the risk of bias tool. However, with only 2 trials identified, this was not performed. The two authors independently extracted risk of bias parameters including: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcomes assessment; incomplete outcome data; selective reporting; and other bias. Outcomes data were extracted, including mean differences in measures of sexual function, desire, and activity. We extracted the data from each study using the tool that the individual studies utilized (for example the composite Brief Index of Sexual Functioning for Women (BISF-W) score as well as mean differences in BISF-W domain scores for thoughts/desire, arousal, frequency of sexual activity, receptivity/initiation, pleasure/orgasm, relationship satisfaction and problems affecting sexual functioning). Overall sexual satisfaction data were also extracted from the instruments used in the individual studies. Data were extracted for the outcomes identified and combined by treatment category to calculate a mean difference and standard error for proportions of successful outcomes. Standard error of the mean was calculated from the equation using standard deviation for each outcome and the total number of patients per treatment arm. We planned to perform a meta-analysis on the identified RCTs but were unable to as the outcomes presented in the identified trials were not reported in a way that allowed for combining. We attempted to contact the trial authors to obtain additional data but did not receive responses.
Results
Trials Included
Only 2 trials met inclusion criteria (Figure 1). These 2 studies included a total of 289 women between ages 20 and 40 years old. Both of these studies investigated the role of bupropion in the treatment of premenopausal women with diagnosed HSDD. More specifically, one of these studies examined women who had all received and failed one or more other previous treatments for HSDD. We searched clinicaltrials.gov to registered ongoing studies using the term “bupropion” and found no ongoing trials for women that would meet our inclusion criteria.
The number of patients randomized into treatment arms of the studies ranged from 31 3 to 116 7. Patients were enrolled for the studies by referral from primary care physician, screened for inclusion via telephone, or sought treatment themselves. Participants were included in the study if they met the criteria of being premenopausal women in committed heterosexual relationships who were not suffering from depression or any other medical condition to explain the etiology of the sexual dysfunction. Health status was established during a screening period through physical examination, thorough medical, sexual and psychosocial history, history of medical therapies, and blood work. Participants in the trials were stratified based on the presence of depressive symptoms. Data were only extracted for those women who were not concurrently depressed.
The Segraves trial, conducted in the United States, randomized 75 women for a period of treatment of 16 weeks. The women were treated with 150mg bupropion for 1 week then the dose increased to 300 mg/day. There was an option to increase the dose to 400mg/d if no response occurred on 300mg. The trial lasted 112 days and women were evaluated with the Changes in Sexual Function Questionnaire (CSFQ) and the Brief Index of Sexual Functioning for Women (BISF-W) at baseline, day 28, 56, 84, and 112. Last observation carried forward was used for missing values 3.
The Safarinejad trial, on the other hand, was a double-blind study conducted in Iran of 232 women randomized into treatment (116) and control (116) groups. Prior to initiation of the trial, all participants were subjected to a 2-week lead-in phase during which they received single-blind placebo in order to screen for response to placebo. Those who demonstrated placebo response in the ‘thoughts/desires’ category were excluded from the trial. The eligible women were then randomized and were given bupropion SR 150 mg daily or placebo. The trial lasted for 12 weeks and participating women were evaluated by completing the BISF-W and Personal Distress Scale (PDS) at baseline and bi-weekly until the end of the trial. The women were also assessed through their answers to a global efficacy question (GEQ; ‘Did the treatment you received during the 12-weeks improve meaningfully your sexual desire?’) 7
The risk of bias present in the allocation was generally unclear for both trials as there was little information provided on methods of randomization or the efforts to maintain concealment (Figure 2). While both trials stated blinding of the participants, only the Safarinejad trial reported additional blinding of the personnel as well as the data assessors. Both studies provided complete record of study attrition in addition to the number of patients reporting to each stage of the trial and successful completion of entire trial. The reported dropout rates were high in the Segraves study, with 35 women dropping out of the study. The Safarinejad study provided complete reporting of participant data assessment and statistical analysis; however, Segraves reported only selective results obtained from participant questionnaires. One trial was supported by the pharmaceutical company that makes bupropion. No explanation of the role of the sponsor was reported 3.
Figure 2.Risk of bias assessment for the included trials. This risk of bias assessment follows the standard guidelines from the Cochrane Handbook (www.cochrane.org)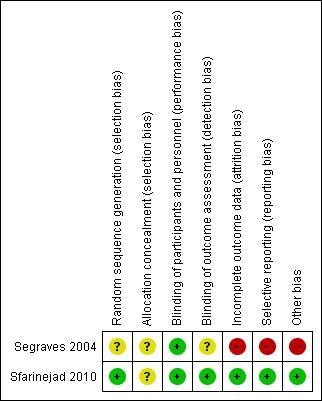
+ = low risk of bias in the domain
? = unclear risk of bias in the domain
- = high risk of bias in the domain
Outcomes
Intention to treat analysis was exercised in both trials, forming groups of 75(randomization) of which 66 returned for analysis 3and 232 participants, of which 223 (96.1%) returned at least once for analysis 7. There were no significant baseline characteristic differences between the treatment and control groups in either trial. Of those that withdrew from the trails, the reasons included lack of efficacy, withdrawal of consent, adverse effects, and unclear loss to follow-up.
The trial performed by Segraves et al. evaluated efficacy in participants on escalating doses of bupropion through the use of CSFQ and BISF-W assessments. The average bupropion dose at the end of the trial (day 112) was 389 mg, during which time there was no evidence of increased efficacy at the higher dose as determined by the CSFQ score. Results from the CSFQ analysis between the bupropion and placebo groups revealed differences in overall total score (F5,260 = 4.99, P = 0.0002), and pleasure (F5,260 = 7.92, P = 0.0001), arousal (F5,260 = 2.41, P = 0.0369), and orgasm (F5,260 = 3.37, P = 0.0057) subdomains. The patients who received bupropion demonstrated higher desire scores, although not statistically significant. The scoring also indicated higher total CSFQ and orgasm scores at the second evaluation (day 28) and sustained this improvement throughout the remainder of the trial. The bupropion-treated participants also demonstrated increases in pleasure subscale as well as statistically significant improvement in the arousal subscale. Assessment by interviewer affirmed statistically greater improvement for positive response to partner initiation in the bupropion group. Analysis of BISF-W scoring did not demonstrate statistical significance; however, the trends of the results indicated improvement over placebo in all variables of the assessment (composite score, thoughts/desires, arousal, frequency of sexual activity, receptivity/initiation, pleasure/orgasm, relationship satisfaction, and problems affecting sexual function). However, these data were presented in prose and figures with p values so individual scores were not extractable.
The Safarinejad, 2010 trial evaluated placebo compared to 150 mg bupropion over a 12 week duration via using the BISF-W, PDS, and global efficacy questions assessments. The composite score on the BISF-W demonstrated significant improvement over the placebo group (15.5 to 16.9 in placebo versus 15.8 to 33.9 in bupropion groups; p=0.001). Overall, there was a statistically significant improvement in all seven of the domains of the BISF-W evaluation and these beneficial effects continued to increase until the 8-week checkpoint. The major effect of bupropion on the BISF-W domains was achieved by week 8 with modest further improvement by 12 weeks and these increases in the scores of the seven BISF-W domains were significantly greater than that seen with placebo. The greatest increases from baseline scores were observed in the domains of frequency of sexual activity, thoughts/desires, and pleasure/orgasm. More specifically, the thoughts/desire score more than doubled in patients treated with bupropion. Additionally, the decrease in PDS scores at 4 weeks was significantly greater than the change seen in the placebo group and continued to be significant through week 12. The response to the GEQ (‘Did the treatment you received during the 12-week trial meaningfully improve your sexual desire?’) revealed statistically significant difference between the bupropion and placebo groups, where 63.5% and 4.3% responded ‘Definitely yes’, respectively (p=0.001). Women who reported a definitely meaningful improvement in sexual desire on the GEQ assessment also had a statistically significantly greater increase from baseline in the BISF-W composite score. Conversely, the participants who reported that they definitely did not experience improvement from treatment additionally demonstrated small and insignificant mean changes from baseline on all seven domains of BISF-W. Lastly, participants were also questioned about overall satisfaction for which 71.8% of the bupropion and 3.7% of placebo groups were definitely satisfied with the efficacy of their treatment (p=0.001). Assessment at the end of the trial revealed that 81% and 34% of sexual episodes were satisfying in the bupropion and placebo groups, respectively (p=0.001). Furthermore, women who reported the greater benefit from bupropion treatment were significantly more likely to express willingness to continue treatment such that 78.1% in the bupropion and 4.9% in the placebo group indicated continuation of therapy (p=0.001).
For both trials, there were very few reported adverse effects, none of which were deemed serious. In the Segraves trial, only 4 participants (6%) withdrew from the study because of side effects 3. In the Safarinejad trial, 28.6% of women taking bupropion and 23.4% of women taking placebo reported adverse events (p=0.03), of which 3 women taking bupropion withdrew from the trial early 7.
Discussion
This systematic review of RCTs reporting the use of bupropion for HSDD in premenopausal women discovered only 2 eligible trials on the topic. Both trials reported that bupropion was an effective and well-tolerated treatment for HSDD in premenopausal women. However, mainly due to potential bias with the Segraves trial and inability to contact the authors to obtain more granular trial data, we were unable to perform a meta-analysis.
Other studies have reported on the use of bupropion for HSDD and other aspects of female sexual dysfunction. A different, single-blind trial by Segraves studied the effects of bupropion in nondepressed women and revealed improvement in libido and overall sexual functioning while being well-tolerated 14. Crenshaw (1987) reported on a population of men and women who experienced improvement in libido and global assessments of sexual function compared to placebo but did not separately report female participants’ results. A trial of minority women who were also depressed showed that switching from an SSRI to bupropion demonstrated significant improvement in desire, arousal, and orgasms (Dobkin 2006). Uncontrolled trials also reported improved subjective measures of HSDD symptoms and emotional satisfaction (Hartmann 2012).
Bupropion has also been used successfully in trials of women with symptoms of female sexual dysfunction believed to be an adverse effect of SSRI administration. These trials show that the addition of or subsequent treatment with bupropion to an SSRI antidepressant regimen results in statistically significant improvement in sexual function, orgasm, and sexual desire in studies using sertraline, fluoxetine, and escitalopram 9, 10, 15, 16, 17. An additional trial conducted by Modell reviewed effects of bupropion on orgasmic dysfunction. These results indicated that bupropion may also be effective in treating orgasmic delay and inhibition as well as disorders of sexual arousal, arguing in support of the prosexual effects of this medication 13.
This systematic review was limited by the lack of RCTs in this area. It is possible that we failed to find a trial on the topic. However, our search strategy and additional searches of reference lists and clinicaltrials.gov are standard means to find other studies. It is unlikely that large, high-quality trial data were missed with our search strategy. We were also unable to contact the Segraves study authors to obtain more detailed data. Thus we were unable to perform a meta-analysis on the commonly reported BISF-W domains. As both trials reported benefit for these domains, combining the data may have strengthened the conclusions of benefit.
As HSDD is a common yet complex problem, multiple solutions have been tested. Based on the data and statistical analysis of these 2 trials, the use of bupropion in premenopausal women with HSDD may be effective in improving sexual dysfunction symptoms, leading to overall improved sexual satisfaction. Treatment of HSDD in premenopausal women with bupropion may hold potential to help women suffering from this condition. More studies are needed to better understand the beneficial impact of the treatment and the potential side effects, particularly with prolonged use. Organizing research resources around this issue that impacts many women and their partners is important. Given the results of this systematic review, there may be potential for bupropion to be used as a treatment for HSDD in premenopausal women.
Conclusions
In conclusion, this systematic review found limited evidence in two small trials demonstrating benefit with bupropion treatment for premenopausal women with HSDD. These findings are preliminary and not of adequate quality to recommend the therapy at this time. More trials are necessary in this area to draw a more concrete conclusion.
References
- 1.Basson R, Brotto L A, Laan E, Redmond G, Utian W H. (2005) . Assessment and Management of Women’s Sexual Dysfunctions: Problematic Desire , Med 2(3), 291-300.
- 2.Laumann E O, Paik A, Rosen R C. (1999) Sexual dysfunction in the united states: Prevalence and predictors. , JAMA 281(6), 537-44.
- 3.Segraves R T, Clayton A, Croft H, Wolf A, Warnock J. (2004) Bupropion Sustained Release for the Treatment of Hypoactive Sexual Desire Disorder in Premenopausal Women. , J. Clin. Psychopharm 24(3), 339-42.
- 4.Graziottin A. (2007) Prevalence and Evaluation of Sexual Health Problems—HSDD in. , Europe. J. Sexual Med 4, 211-9.
- 5.. American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4th ed., text revision. ed. Washington, DC ::American Psychiatric Association;2000 .
- 6.Segraves K B, Segraves R T. (1991) Hypoactive Sexual Desire Disorder: Prevalence and Comorbidity in 906 Subjects. , J. Sex & Marital Therapy 17(1), 55-8.
- 7.Safarinejad M R, Hosseini S Y, Asgari M A, Dadkhah F, Taghva A.A randomized, double-blind, placebo-controlled study of the efficacy and safety of bupropion for treating hypoactive sexual desire disorder in ovulating women. , BJU Int.2010Sep; 106(6), 832-9.
- 8.A H Clayton, J F Pradko, H A Croft, C B Montano, R A Leadbetter et al. (2002) Prevalence of sexual dysfunction among newer antidepressants. , J. Clin. Psych 63(4), 357-66.
- 9.Clayton A H, Warnock J K, Kornstein S G, Pinkerton R, Sheldon-Keller A et al. (2004) A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. , J. Clin. Psych 65(1), 62-7.
- 10.P S Masand, A K, Gupta S, Frank B.Sustained-release bupropion for selective serotonin reuptake inhibitor-induced sexual dysfunction: a randomized, double-blind, placebo-controlled, parallel-group study. , Am. J. Psych.2001May; 158(5), 805-7.
- 11.Croft H, Jr Settle, Houser E, Batey T, S R Donahue et al. (1999) A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. , Clin. Ther 21(4), 643-58.
- 12.T L Crenshaw, J P Goldberg, W C Stern.Pharmacologic modification of psychosexual dysfunction. , J. Sex Marital Ther.1987Winter; 13(4), 239-52.
- 13.Modell J M R K C. (2000) Effect of Bupropion-SR on Orgasmic Dysfunction in Nondepressed Subjects: A Pilot Study. , J. Sex & Marital Ther 26(3), 231-40.
- 14.Segraves R C, Kavoussi H, Ascher R, Batey J A, VJ Bolden-Watson Foster et al. (2001) Bupropion Sustained Release (SR) for the Treatment of Hypoactive Sexual Desire Disorder (HSDD) in Nondepressed Women. , J. Sex & Marital Ther 27(3), 303-16.
- 15.Taylor M J R, Bullemor-Day P, Lubin J, Chukwujekwu C, Hawton K. (2013) Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database of Systematic Reviews. 5.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
Current Neuropharmacology (2022) Crossref
Current Neuropharmacology (2022) OpenAlex
Journal of Affective Disorders (2019) Crossref
Journal of Affective Disorders (2019) OpenAlex
Yifeng Shen, Qian Zhao, Yi-min Yu, Yunlong Tan, Honggeng Zhang et al. - Journal of Affective Disorders (2019) Semantic Scholar
