Estimation of Glycemic Index of Liver Nutritional Supplement and its Importance in Liver Nutrition
Abstract
A global increase in incidence of chronic liver disease (CLD) indicated the necessity of dietary and lifestyle modification. Low glycemic index (GI) diet was reported to have a significant role in controlling diabetes caused by liver dysfunction. The International Standards Organisation (ISO) has standardized the determination of GI of a food in healthy individuals. This study aimed to estimate GI value of a high protein, energy dense liver nutritional supplement. This cross-over randomized controlled study randomly allotted 15 participants to consume either reference food 27.5 gm glucose (glucose monohydrate) or 77 gm nutritional supplement (equivalent to 25 gm of available carbohydrates); switching to another arm was done after 3 days wash-out period. After overnight fast, blood samples were collected at 15, 30, 45 and 60 minutes post-consumption of s upplement or reference food. The GI was calculated from the incremental area under the blood glucose response elicited by the nutritional supplement as a percentage of the response after consumption of 27.5 gm of glucose (glucose monohydrate) by the same participant using a standard formula. Mean GI of the nutritional supplementwas estimated as 11.4 ± 2.4.With the consumption of this nutritional supplement, the blood glucose levels were reduced at all postprandial time points, compared to the reference food. The liver nutritional supplement tested has a low GI, and comparatively slower and more sustained blood glucose response. Therefore, it can be used in patients with CLD to prevent CLD-associated metabolic complications and improve health outcomes and quality of life.
Author Contributions
Academic Editor: Jong In Kim, Korea, Republic of.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 Rachana Bhoite, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Chronic liver disease (CLD) results in protein-calorie malnutrition, which can be observed in 65-90% of patients with advanced CLD 1, 2. Between 1980-2013, 46% increase in CLD mortality was reported, especially in the low and low-middle income countries of Asia and Africa 3. Nonalcoholic fatty liver disease (NAFLD) is the most common form of CLD, ranging from steatosis to steatohepatitis, and is characterized by obesity, insulin resistance, and dyslipidemia; whereas cirrhosis is the final stage of CLD, characterized by liver structure disruption and widespread nodule formation 4, 5. Besides unhealthy lifestyle, another reason for NAFLD prognosis is insulin resistance-induced hyperglycemia that affects macronutrient metabolism, resulting in further development of steatohepatitis, cirrhosis and hepatocellular carcinomas in worst-case scenario 6. The American Diabetes Association recommends weight loss by modifications in diet habit, focusing more on carbohydrate source, fiber, and glycemic index (GI) of the food, along with pharmacotherapy for management of CLD 7. Patients with liver disease must aim to reduce 5-10% body weight by opting a moderate carbohydrate diet (40-65% of energy), along with consumption of limited saturated and trans fat, fructose, and simple carbohydrates 4, 8. Generally, patient with NAFLD consumes carbohydrate foods with high GI,which is associated with insulin resistance, and metabolic and liver dysfunction 9, as well as higher risk of mortality 10.The carbohydrate content of a food is measured by GI value, typically after consuming a carbohydrate containing test food relative to a carbohydrate containing reference food (glucose or white bread). The International Standards Organisation (ISO 26642-2010) has standardized the determination of GI (low, medium or high GI) of carbohydrates in foods 11. Limiting high GI diet in NAFLD patients has shown beneficial effects 9 as it elicits a low postprandial glucose response and prevents development of hepatogenous diabetes, i.e., diabetes caused by liver dysfunction in CLD patients 4, 8.
A high protein and energy dense nutritional supplement with added branched-chain amino acids (BCAA) is designed especially for patients with CLD to maintain an adequate calorie and protein intake. The supplement may help individuals with CLD to manage their postprandial glucose levels, and to improve immunity. A cross-over randomized controlled study was designed to estimate the GI value of this liver nutritional supplement in healthy individuals.
Materials and Methods
This study was conducted using internationally recognized GI protocol by FAO/WHO, recommended guidelines by International dietary carbohydrate task force for GI methodology 12, and International Standards Organisation (ISO 26642-2010) 11which have been validated and published 13. The procedure used in this study was in accordance with international standards for conducting ethical research with humans and was approved by the institutional ethics committee of Madras Diabetes Research Foundation (MDRF), Chennai, India. The study was registered in the clinical trial registry of India, CTRI/2021/08/035929, and designed according to the Consolidated Standards of Reporting Trials (CONSORT). Informed consent was obtained from all study participants.
Study Design and Study Participants
In this cross-over randomized controlled study, healthy male or female (n=15) volunteers, aged between 18 and 45 years, with body mass index (BMI) ≤ 22.9 kg/m2, who were willing to consume the nutritional supplement and reference food, were recruited from the participant roster of Glycemic Index Testing Centre of MDRF. Participants were excluded from the study based on the following criteria: if they were on a specific diet restriction; or pregnant and lactating; or had a known history of diabetes mellitus; or presence of any disease or drug(s), which may influence digestion and absorption of nutrients; or any major medical/ surgical event in the last 3 months.
Study Intervention/ Dietary Intervention
The nutritional supplement is a blend of whey protein isolates, soy protein, BCAA, medium chain triglycerides, and vitamins and minerals.
Following the GI protocol, 77 gm of the nutritional supplement (to get 25 gm of available carbohydrates) was mixed with 334 ml of water and given to all study participants along with 125 ml of plain water. As the reference food, 27.5 gm of glucose (glucose monohydrate) dissolved in 125 ml of water was given to the study participants. The GI value of the nutritional supplement was calculated as follows:
Outcome Measurements
The volunteers were instructed to visit the center on each test day in the morning after 10-12 hours of overnight fasting. They underwent 3 days of testing with the reference food and 2 days with the nutritional supplement in random order with 3 days of wash-out period between measurements to minimize carry-over effects (Figure 1). The 24 hours dietary recall was recorded. Height (cm) and weight (Kg) were measured. Body mass index (BMI) was calculated using the formula: weight (kg)/ height (m2). In addition, at baseline, the socio-demographics of the participants along with details of physical activity, smoking and alcohol, and consumption of caffeine containing drinks were registered via brief questionnaire.
Figure 1.Flow chart for intervention
Blood samples were drawn by trained medical staff for determination of GI. Before consumption of the food, the fasting blood samples were taken at -5 minutes and 0 minute by finger-prick using an automatic lancet device; the baseline value was taken as the mean of these two values. The participants then consumed 25 gm of available carbohydrate portion of the nutritional supplement: the first bite/sip in the mouth was set as time 0 and the first blood sample was taken exactly after 15 minutes. The capillary blood samples were obtained at 30, 45, 60, 90 and 120 minutes after the start of the test meal for glucose estimations. Participants were given 125 ml of water during the subsequent 2 hours.
Statistical Analysis
Descriptive statistics for baseline socio-demographics, GI value and metabolic measures were computed and examined. Mean and standard error of mean are presented as mean ± Standard Error of the Mean (SEM). The incremental area under the curve (IAUC) of blood glucose for the reference food and nutritional supplement were calculated geometrically using the trapezoid rule, ignoring the area below the fasting baseline, and expressed as mean ± SEM. The GI values were calculated by expressing each subject’s IAUC after consumption of nutritional supplement as the percentage of the same subject’s mean reference IAUC (mean ± SEM). The group mean of the resulting value was declared as the GI of the nutritional supplement. All analyses were performed using SPSS 27.0 (SPSS, Chicago, Illinois, USA) software.
Results
Participant Characteristics
A total of 15 healthy volunteers with mean age of 27.5 ± 1.2 years, and mean BMI of 20.9 ± 0.3 kg/m2 were included in the study. Out of these, one subject with CV > 30% and another subject with GI value > mean GI ± 2SD were removed as outliers, following the ISO protocol 11. Further 3 participants dropped out from the study for personal reasons. Therefore, the data of the remaining 10 subjects were analyzed. The baseline characteristics of these study participants are given inTable 1
Table 1. Baseline characteristics of the study participants| Subject Characteristics | Study group (n=15) |
| Age (years) | 27.5 ± 1.2 |
| Gender, n (%) | |
| Male | 7 (47.0) |
| Female | 8 (53.0) |
| Weight (Kg) | 55.0 ± 2.0 |
| BMI (Kg/m2) | 20.9 ± 0.3 |
| Fasting blood glucose (mg/dl) | 86.0 ± 2.0 |
Determination of Glycemic Index
The mean IAUC of reference food and nutritional supplement, and the GI of the nutritional supplement are presented in Table 2.
Table 2. Individual mean IAUC of reference (glucose) and GI of nutritional supplement| Participant No. | IAUC of reference food (mg/dl ¥ min) (n=15) | IAUC of nutritional supplement (mg/dl ¥ min) (n=15) | GI of nutritional supplement (n=15) |
| 1 | 5119.1 | 522.1 | 10.2 |
| 2 | 3620.9 | 521.3 | 14.4 |
| 3 | 5227.4 | * | * |
| 4 | 3983 | ** | ** |
| 5 | 1719 | 38 | 2.2 |
| 6 | 2352.5 | 21 | 0.9 |
| 7 | 2367 | 566.3 | 23.9 |
| 8 | 3072.1 | 132.9 | 4.3 |
| 9 | 3015.2 | 567.2 | 18.8 |
| 10 | 3471.5 | 299.1 | 8.6 |
| 11 | 3719.4 | *** | *** |
| 12 | 3653.2 | 479 | 13.1 |
| 13 | 2623.7 | ** | ** |
| 14 | 3127.6 | 547.5 | 17.5 |
| 15 | 1274.3 | ** | ** |
| Mean ± SEM | 3223.1 ± 283.1 | 369.4 ± 71 | 11.4 ± 2.4 |
|---|
The minimum and maximum values of GI in the nutritional supplement were recorded as 0.9 and 18.8 respectively, and the mean GI was estimated to be 11.4 ± 2.4. In addition, the nutritional supplement has produced much lower blood glucose levels, compared to the reference food at 15, 30, 45 and 60 minutes post-consumption (Figure 2).
Figure 2.Comparison of reference food (glucose) with nutritional supplement in terms of changes in blood glucose over a period of two hours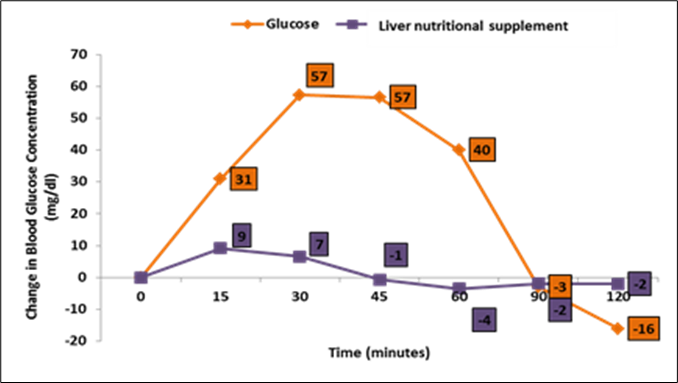
Discussion
The growing incidence of CLD all over the world stresses the urgent need for dietary and lifestyle modifications. The knowledge of GI of a food is necessary to make healthy food choices. There are evidences, suggesting beneficial role of low GI diet in overweight/obese patients 14, and patients with NAFLD 8, 9. As far as we are concerned this is the first study to assess the GI value of a newly launched liver nutritional supplement in healthy individuals, which is a protein and energy dense food with added BCAA, vitamins and minerals. In addition, change in blood glucose level after consumption of this food relative to consumption of glucose was also compared. The GI of this n utritional supplement was estimated as 11.4 ± 2.4 which falls within the ‘low GI category’. Moreover, there was a lower blood sugar response with this supplement, compared to the reference food, throughout the postprandial period.
The association between CLD and diabetes mellitus is known since long, and consumption of low GI diet helps to control CLD-induced hepatogenous diabetes and protein calorie malnutrition 15. In our previous study, we have assessed the GI and glycemic response of a plant-based supplement, and found that low GI diet has improved satiety and can be included in the diet to reduce the postprandial glycemic response in overweight and obese people 14. Recently, a significant reduction in hepatic mass in individuals without NAFLD is also reported with low GI diet 9. In a previous randomized cross-over study, healthy males have participated in two 7 day high GI and low GI diets, separated by a 4 week wash-out period. In this study, an increase in fasted fat fractions after high GI diet and a decrease in fasted fat fractions after low GI diet were observed compared to baseline. For the low GI diet, significant reduction in fat fractions at 360 minutes after test meal was also noticed 16.
In a randomized, double-blind, parallel design diet intervention study, the effects of a low-fat/ low saturated fat/ low GI diet (LSAT) was compared to a high-fat/ high saturated fat/ low GI diet in older adults over a 4 week period. Compared to baseline, LSAT showed significant reduction in liver fat percentage 17. The findings were also consistent with another cross-sectional study, in which a strong correlation between higher GI foods and high-grade liver stenosis, particularly among individuals with insulin resistance was recorded 18. Furthermore, the liver biomarker, alanine transaminase was reported to be significantly improved in obese individuals with type 2 diabetes after consuming low GI diet along with a modified Mediterranean diet 19.
Patients with CLD have a tendency to suffer from fat-soluble vitamin deficits, early recognition of which is essential to reduce the chances of infection, in-hospital mortality and to improve the liver function 2. Therefore, modifications in the diet to compensate the nutrient deficiency and to make healthy lifestyle choices are highly recommended in order to control plasma glucose level and CLD prognosis. The consumption of low GI foods may have a beneficial impact on the society as this can help to enhance the quality of life, health and well-being of the patient by reducing the burden of disease progression to advanced liver diseases. This in turn emphasizes the need for developing low GI nutritional supplement with ingredients, beneficial for liver health.
Conclusion
Mean GI of the liver nutritional supplement was estimated as 11.4 ± 2.4 which is below 55, hence has a low GI. The supplement is high in protein and is energy dense, it has slower and more sustained blood glucose response compared to glucose. Therefore, the supplement can be used as an adjuvant to treatment in patients with CLD to prevent CLD-associated metabolic complications and improve overall health outcomes and quality of life.
Funding
This research was funded by Dr. Reddy’s Laboratories Ltd.
Compliance with Ethical Standards
Institutional review board statement: The study was reviewed and approved by the institutional ethics committee of Madras Diabetes Research Foundation, Chennai, India.
Clinical trial registration statement: The study was registered in the clinical trial registry of India. The registration identification number is CTRI/2021/08/035929.
Informed consent statement: Prior to the study, informed consent was obtained from all study participants.
Acknowledgements
Authors thank WorkSure® for manuscript writing support.
References
- 1.Sharabi K, CDJ Tavares, Rines A K, Puigserver P. (2015) . Molecular Pathophysiology of Hepatic Glucose Production. DOI: 10.1016/j.mam.2015.09.003. Molecular aspects of medicine 46, 21-33.
- 2.Silva M, Gomes S, Peixoto A, Torres-Ramalho P, Cardoso H. (2015) . Nutrition in Chronic Liver Disease. DOI: 10.1016/j.jpge.2015.06.004. GE Portuguese journal of gastroenterology 22(6), 268-276.
- 3.Mukherjee P S, Vishnubhatla S, Amarapurkar D N, Das K, Sood A. (2017) Etiology and mode of presentation of chronic liver diseases in India: A multi centric study. DOI: 10.1371/journal.pone.0187033. PLoS One 12, 1-13.
- 4.Conlon B A, Beasley J M, Aebersold K, Jhangiani S S, Wylie-Rosett J. (2013) . Nutritional management of insulin resistance in nonalcoholic fatty liver disease (NAFLD). DOI: 10.3390/nu5104093. Nutrients 5, 4093-114.
- 5.Sharma A, Nagalli S. (2022) Chronic liver disease. In StatPearls. StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK554597/.
- 6.Mohamed J, AHN Nafizah, Budin S B. (2016) Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. DOI: 10.18295/squmj.2016.16.02.002. Sultan Qaboos University medical journal 16(2), 132-141.
- 7.American Diabetes Association (2017) Standards of Medical Care. in Diabetes-2017 Abridged for Primary Care Providers. DOI: 10.2337/cd16-0067. Clinical diabetes : a publication of the American Diabetes Association 35(1), 5-26.
- 8.Papamiltiadous E S, Roberts S K, Nicoll A J, Ryan M C, Itsiopoulos C. (2016) A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): study protocol. , BMC Gastroenterology 16, 10-1186.
- 9.Al-Awadi A, Grove J, Taylor M, Valdes A, Vijay A. (2021) Effects of an isoenergetic low Glycaemic Index (GI) diet on liver fat accumulation and gut microbiota composition in patients with non-alcoholic fatty liver disease (NAFLD): A study protocol of an efficacy mechanism evaluation. , DOI: 10.1136/bmjopen-2020-045802. BMJ Open 11(10).
- 10.DJA Jenkins, Dehghan M, Mente A, Bangdiwala S I, Rangarajan S. (2021) . Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. DOI: 10.1056/NEJMc2107926. The New England journal of medicine 385(4), 379-380.
- 11. (2010) International Organization for Standardization. Food products--Determination of the glycaemic index (GI) and recommendation for food classification.
- 12.Brouns F, Bjorck I, Frayn K N, Gibbs A L, Lang V. (2005) . Glycaemic index methodology. DOI: 10.1079/NRR2005100. Nutrition research reviews 18(1), 145-171.
- 13.CJK Henry, Lightowler H J, Newens K, Sudha V, Radhika G. (2008) . Glycaemic index of common foods tested in the UK and India. DOI: 10.1017/S0007114507831801The British journal of nutrition 99(4), 840-845.
- 14.Rachana B, Vijayalakshmi P, Ganesh R J, Gopinath V, Parkavi K. (2019) Glycemic Index and Response of a Plant Based Nutritional Supplement and Its Subjective Satiety Following Its Use in Indian Adults. DOI: 10.4236/fns.2019.108067. Food and Nutrition Sciences 10, 937-946.
- 15.Kumar R. (2018) Hepatogenous diabetes: An underestimated problem of liver cirrhosis. DOI: 10.4103/ijem.IJEM_79_18. Indian journal of endocrinology and metabolism 22(4), 552-559.
- 16.Bawden S, Stephenson M, Falcone Y, Lingaya M, Ciampi E. (2017) Increased liver fat and glycogen stores after consumption of high versus low glycaemic index food: A randomized crossover study. Diabetes, DOI: 10.1111/dom.12784. Diabetes, Obesity and Metabolism 19, 70-7.
- 17.Utzschneider K M, Bayer-Carter J L, Arbuckle M D, Tidwell J M, Richards T L. (2013) Beneficial effect of a weight-stable, low-fat/low-saturated fat/low-glycaemic index diet to reduce liver fat in older subjects. DOI: 10.1017/S000711451200296The British journal of nutrition 109(6), 1096-1104.
- 18.Valtueña S, Pellegrini N, Ardigò D, Del Rio D, Numeroso F. (2006) Dietary glycemic index and liver steatosis. DOI: 10.1093/ajcn/84.1.136. The American journal of clinical nutrition. 84(1), 136-269.
- 19.Fraser A, Abel R, Lawlor D A, Fraser D, Elhayany A. (2008) A modified Mediterranean diet is associated with the greatest reduction in alanine aminotransferase levels in obese type 2 diabetes patients: Results of a quasi-randomised controlled trial. , DOI: 10.1007/s00125-008-1049-1. Diabetologia 51(9), 1616-22.